Content from Introducing R and RStudio IDE
Last updated on 2023-08-15 | Edit this page
Estimated time 45 minutes
Overview
Questions
- Why use R?
- Why use RStudio and how does it differ from R?
Objectives
- Know advantages of analyzing data in R
- Know advantages of using RStudio
- Create an RStudio project, and know the benefits of working within a project
- Be able to customize the RStudio layout
- Be able to locate and change the current working directory with
getwd()andsetwd() - Compose an R script file containing comments and commands
- Understand what an R function is
- Locate help for an R function using
?,??, andargs()
Getting ready to use R for the first time
In this lesson we will take you through the very first things you need to get R working.
Tip: This lesson works best on the cloud
Remember, these lessons assume we are using the pre-configured virtual machine instances provided to you at a genomics workshop. Much of this work could be done on your laptop, but we use instances to simplify workshop setup requirements, and to get you familiar with using the cloud (a common requirement for working with big data). Visit the Genomics Workshop setup page for details on getting this instance running on your own, or for the info you need to do this on your own computer.
A Brief History of R
R has been around since 1995, and was created by Ross Ihaka and Robert Gentleman at the University of Auckland, New Zealand. R is based off the S programming language developed at Bell Labs and was developed to teach intro statistics. See this slide deck by Ross Ihaka for more info on the subject.
Advantages of using R
At more than 20 years old, R is fairly mature and growing in popularity. However, programming isn’t a popularity contest. Here are key advantages of analyzing data in R:
- R is open source. This means R is free - an advantage if you are at an institution where you have to pay for your own MATLAB or SAS license. Open source, is important to your colleagues in parts of the world where expensive software in inaccessible. It also means that R is actively developed by a community (see r-project.org), and there are regular updates.
- R is widely used. Ok, maybe programming is a popularity contest. Because, R is used in many areas (not just bioinformatics), you are more likely to find help online when you need it. Chances are, almost any error message you run into, someone else has already experienced.
- R is powerful. R runs on multiple platforms (Windows/MacOS/Linux). It can work with much larger datasets than popular spreadsheet programs like Microsoft Excel, and because of its scripting capabilities is far more reproducible. Also, there are thousands of available software packages for science, including genomics and other areas of life science.
Introducing RStudio Server
In these lessons, we will be making use of a software called RStudio, an Integrated Development Environment (IDE). RStudio, like most IDEs, provides a graphical interface to R, making it more user-friendly, and providing dozens of useful features. We will introduce additional benefits of using RStudio as you cover the lessons. In this case, we are specifically using RStudio Server, a version of RStudio that can be accessed in your web browser. RStudio Server has the same features of the Desktop version of RStudio you could download as standalone software.
Log on to RStudio Server
Open a web browser and enter the URL you used to log in at the
terminal (provided by your instructors), followed by :8787.
For example, if your URL was ec2.12.2.45.678.compute-1.amazonaws.com,
you should enter:
http://ec2.12.2.45.678.compute-1.amazonaws.com:8787You should now be looking at a page that will allow you to login to the RStudio server:
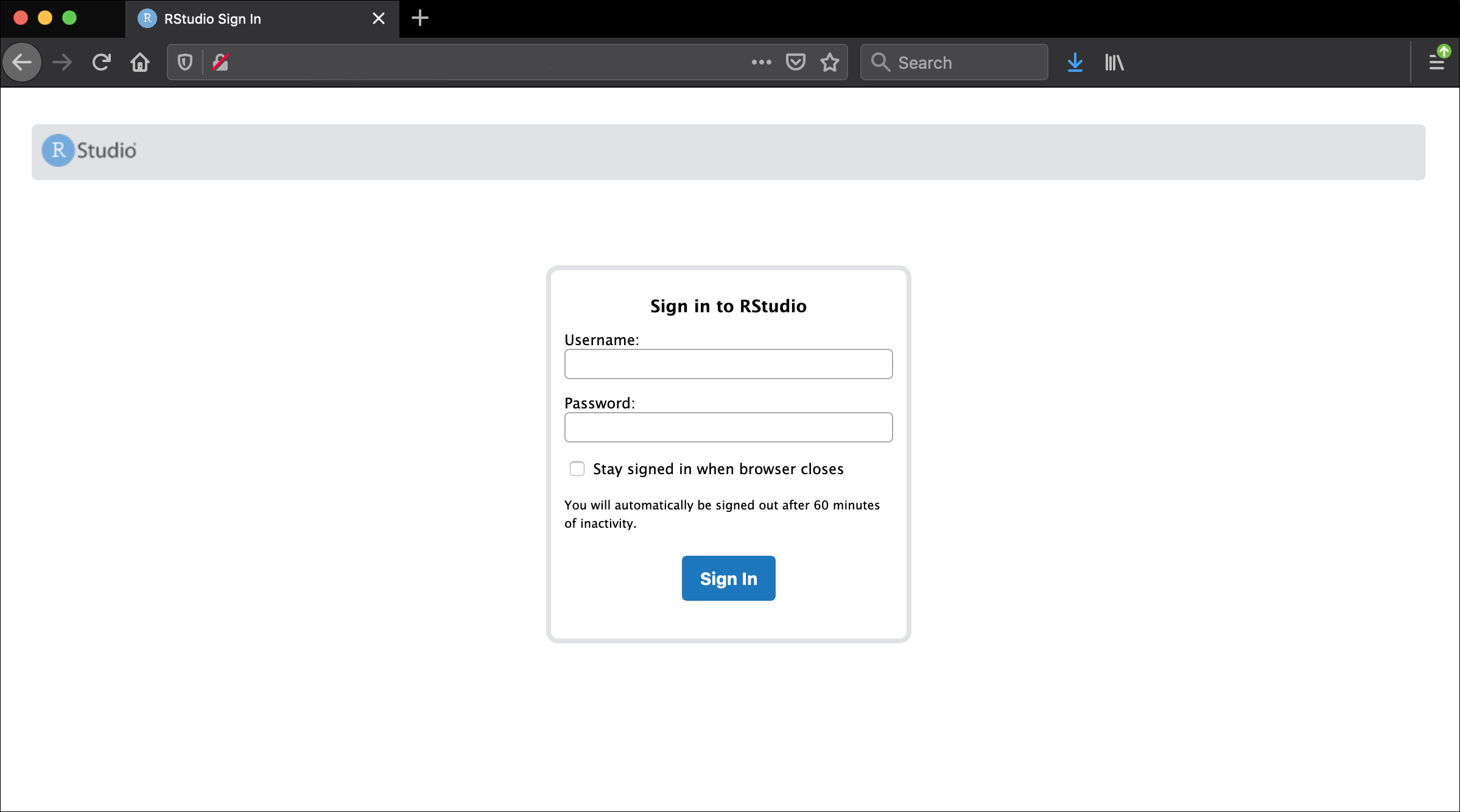
Enter your user credentials and click Sign In. The credentials for the genomics Data Carpentry instances will be provided by your instructors.
You should now see the RStudio interface:
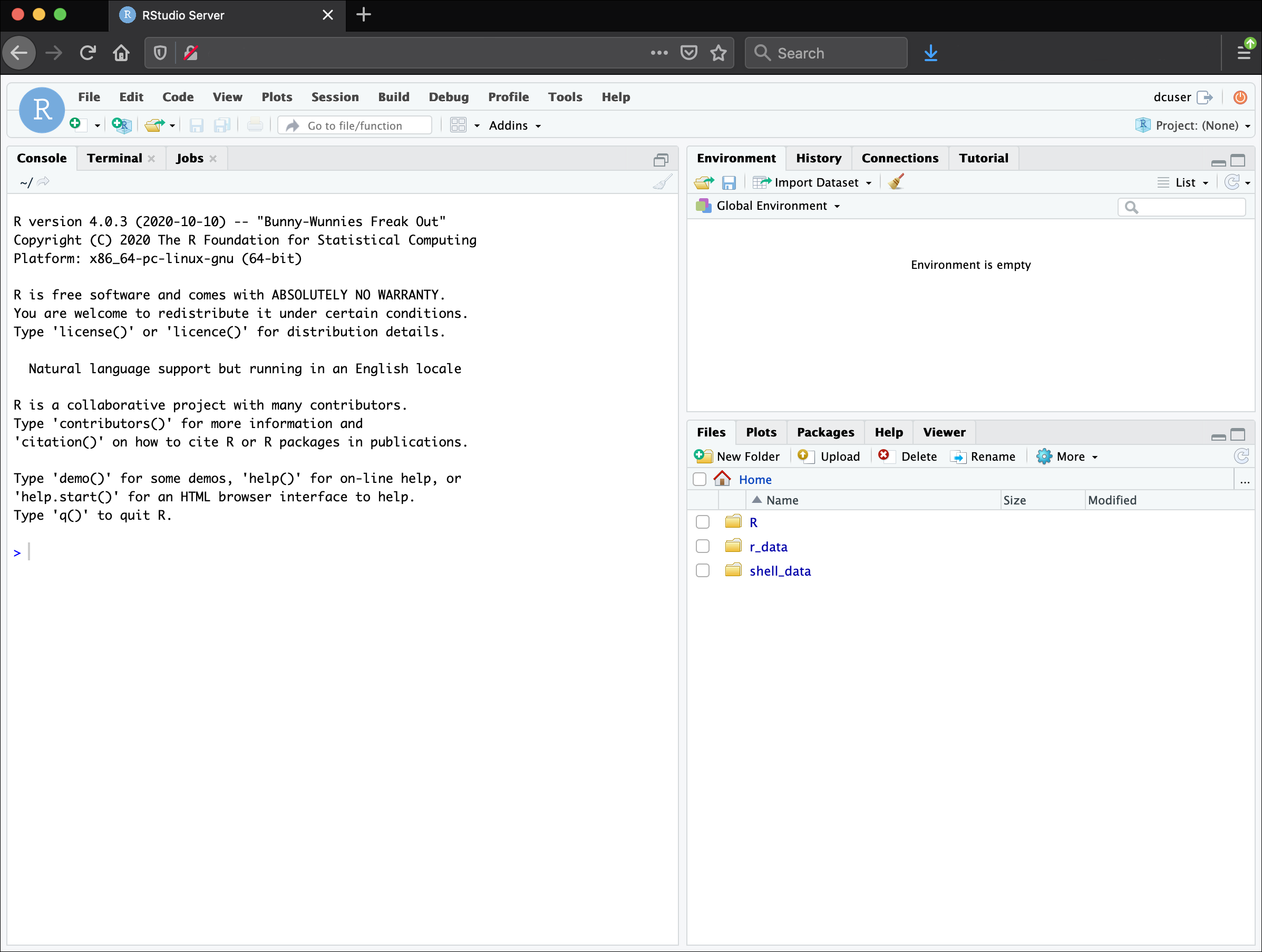
Create an RStudio project
One of the first benefits we will take advantage of in RStudio is something called an RStudio Project. An RStudio project allows you to more easily:
- Save data, files, variables, packages, etc. related to a specific analysis project
- Restart work where you left off
- Collaborate, especially if you are using version control such as git.
- To create a project, go to the File menu, and click New Project….
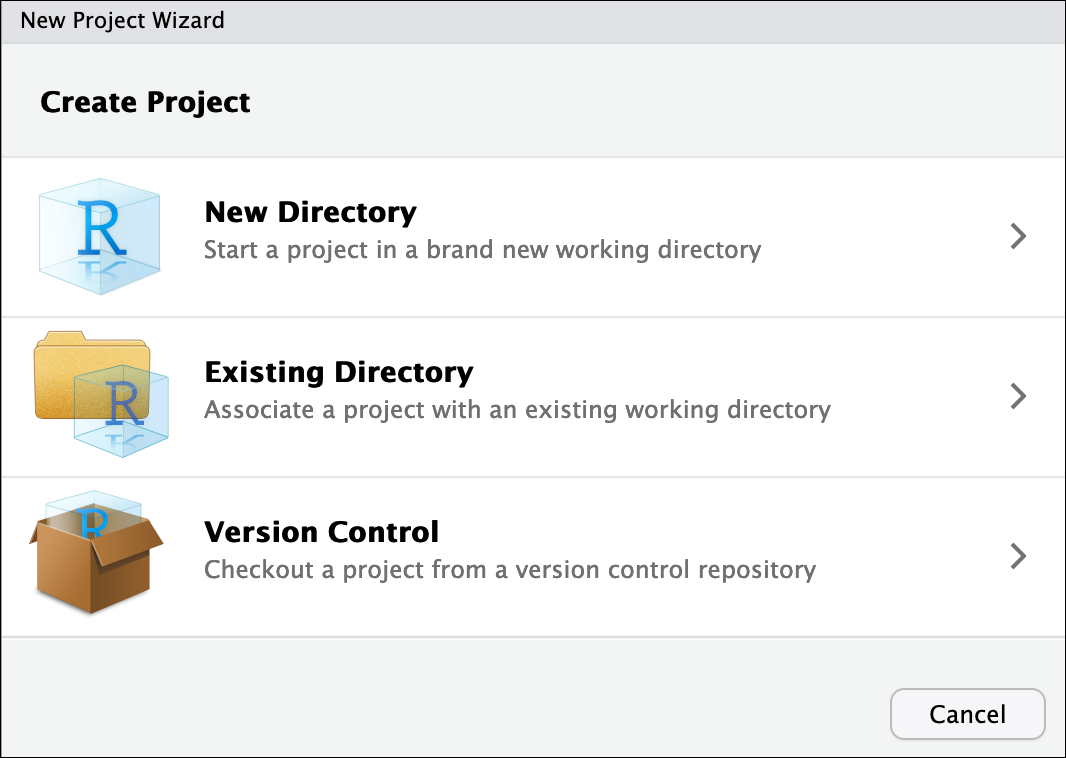
In the window that opens select New Directory, then New Project. For “Directory name:” enter dc_genomics_r. For “Create project as subdirectory of”, click Browse… and then click Choose which will select your home directory “~”.
Finally click Create Project. In the “Files” tab of your output pane (more about the RStudio layout in a moment), you should see an RStudio project file, dc_genomics_r.Rproj. All RStudio projects end with the “.Rproj” file extension.
Tip: Make your project more reproducible with renv
One of the most wonderful and also frustrating aspects of working with R is managing packages. We will talk more about them, but packages (e.g. ggplot2) are add-ons that extend what you can do with R. Unfortunately it is very common that you may run into versions of R and/or R packages that are not compatible. This may make it difficult for someone to run your R script using their version of R or a given R package, and/or make it more difficult to run their scripts on your machine. renv is an RStudio add-on that will associate your packages and project so that your work is more portable and reproducible. To turn on renv click on the Tools menu and select Project Options. Under Enviornments check off “Use renv with this project” and follow any installation instructions.
Creating your first R script
Now that we are ready to start exploring R, we will want to keep a record of the commands we are using. To do this we can create an R script:
Click the File menu and select New File and then R Script. Before we go any further, save your script by clicking the save/disk icon that is in the bar above the first line in the script editor, or click the File menu and select save. In the “Save File” window that opens, name your file “genomics_r_basics”. The new script genomics_r_basics.R should appear under “files” in the output pane. By convention, R scripts end with the file extension .R.
Overview and customization of the RStudio layout
Here are the major windows (or panes) of the RStudio environment:
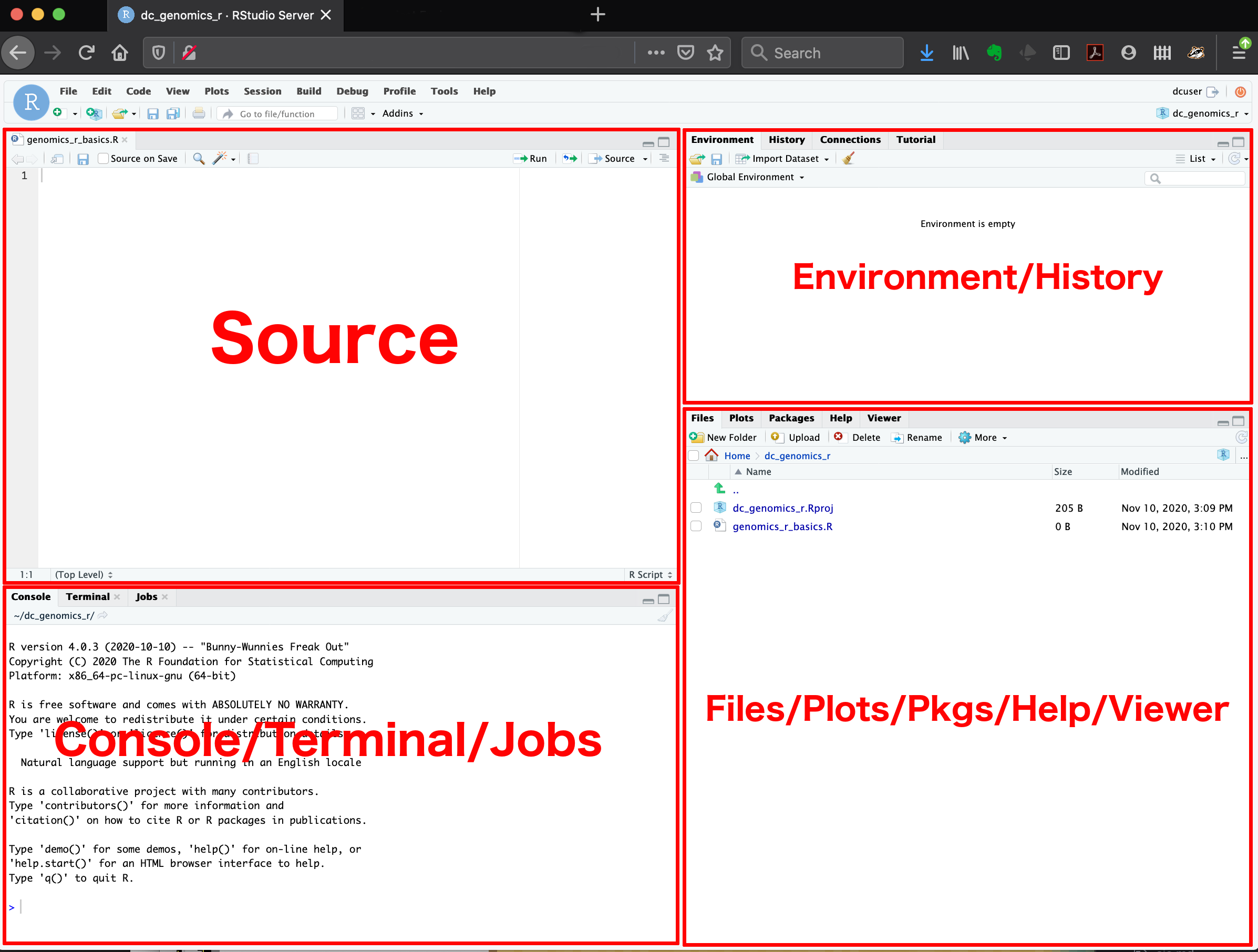
-
Source: This pane is where you will write/view R
scripts. Some outputs (such as if you view a dataset using
View()) will appear as a tab here. - Console/Terminal/Jobs: This is actually where you see the execution of commands. This is the same display you would see if you were using R at the command line without RStudio. You can work interactively (i.e. enter R commands here), but for the most part we will run a script (or lines in a script) in the source pane and watch their execution and output here. The “Terminal” tab give you access to the BASH terminal (the Linux operating system, unrelated to R). RStudio also allows you to run jobs (analyses) in the background. This is useful if some analysis will take a while to run. You can see the status of those jobs in the background.
- Environment/History: Here, RStudio will show you what datasets and objects (variables) you have created and which are defined in memory. You can also see some properties of objects/datasets such as their type and dimensions. The “History” tab contains a history of the R commands you’ve executed R.
- Files/Plots/Packages/Help/Viewer: This multipurpose pane will show you the contents of directories on your computer. You can also use the “Files” tab to navigate and set the working directory. The “Plots” tab will show the output of any plots generated. In “Packages” you will see what packages are actively loaded, or you can attach installed packages. “Help” will display help files for R functions and packages. “Viewer” will allow you to view local web content (e.g. HTML outputs).
All of the panes in RStudio have configuration options. For example, you can minimize/maximize a pane, or by moving your mouse in the space between panes you can resize as needed. The most important customization options for pane layout are in the View menu. Other options such as font sizes, colors/themes, and more are in the Tools menu under Global Options.
You are working with R
Although we won’t be working with R at the terminal, there are lots of reasons to. For example, once you have written an RScript, you can run it at any Linux or Windows terminal without the need to start up RStudio. We don’t want you to get confused - RStudio runs R, but R is not RStudio. For more on running an R Script at the terminal see this Software Carpentry lesson.
Using functions in R, without needing to master them
A function in R (or any computing language) is a short program that
takes some input and returns some output. Functions may seem like an
advanced topic (and they are), but you have already used at least one
function in R. getwd() is a function! The next sections
will help you understand what is happening in any R script.
-
dir()# Lists files in the working directory -
sessionInfo()# Gives the version of R and additional info including on attached packages -
date()# Gives the current date -
Sys.time()# Gives the current time
Notice: Commands are case sensitive!
You have hopefully noticed a pattern - an R function has three key properties:
- Functions have a name (e.g.
dir,getwd); note that functions are case sensitive! - Following the name, functions have a pair of
() - Inside the parentheses, a function may take 0 or more arguments
An argument may be a specific input for your function and/or may
modify the function’s behavior. For example the function
round() will round a number with a decimal:
R
# This will round a number to the nearest integer
round(3.14)
OUTPUT
[1] 3Getting help with function arguments
What if you wanted to round to one significant digit?
round() can do this, but you may first need to read the
help to find out how. To see the help (In R sometimes also called a
“vignette”) enter a ? in front of the function name:
R
?round()
The “Help” tab will show you information (often, too much
information). You will slowly learn how to read and make sense of help
files. Checking the “Usage” or “Examples” headings is often a good place
to look first. If you look under “Arguments,” we also see what arguments
we can pass to this function to modify its behavior. You can also see a
function’s argument using the args() function:
R
args(round)
OUTPUT
function (x, digits = 0)
NULLround() takes two arguments, x, which is
the number to be rounded, and a digits argument. The
= sign indicates that a default (in this case 0) is already
set. Since x is not set, round() requires we
provide it, in contrast to digits where R will use the
default value 0 unless you explicitly provide a different value. We can
explicitly set the digits parameter when we call the function:
R
round(3.14159, digits = 2)
OUTPUT
[1] 3.14Or, R accepts what we call “positional arguments”, if you pass a
function arguments separated by commas, R assumes that they are in the
order you saw when we used args(). In the case below that
means that x is 3.14159 and digits is 2.
R
round(3.14159, 2)
OUTPUT
[1] 3.14Finally, what if you are using ? to get help for a
function in a package not installed on your system, such as when you are
running a script which has dependencies.
R
?geom_point()
will return an error:
ERROR
Error in .helpForCall(topicExpr, parent.frame()) :
no methods for ‘geom_point' and no documentation for it as a functionUse two question marks (i.e. ??geom_point()) and R will
return results from a search of the documentation for packages you have
installed on your computer in the “Help” tab. Finally, if you think
there should be a function, for example a statistical test, but you
aren’t sure what it is called in R, or what functions may be available,
use the help.search() function.
While your search results may return several tests, we list a few you might find:
- Chi-Squared test:
stats::Chisquare - Student t-test:
stats::t.test - mixed linear model:
stats::lm.glm
We will discuss more on where to look for the libraries and packages that contain functions you want to use. For now, be aware that two important ones are CRAN - the main repository for R, and Bioconductor - a popular repository for bioinformatics-related R packages.
RStudio contextual help
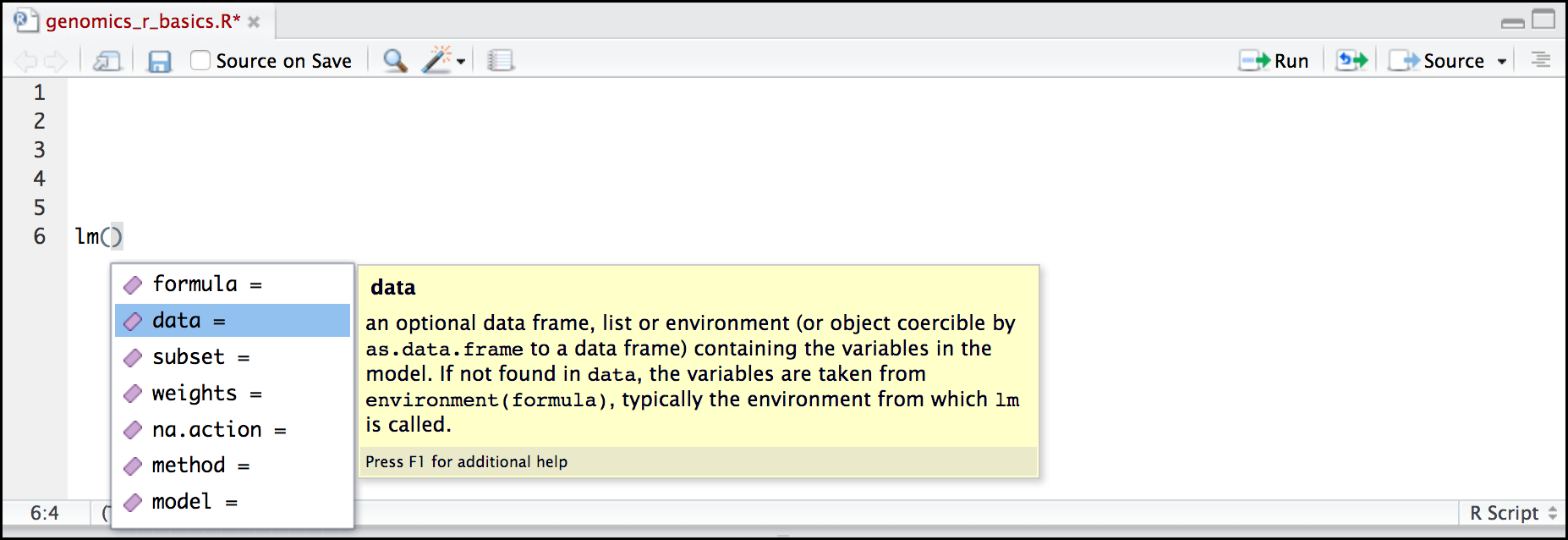
Here is one last bonus we will mention about RStudio. It’s difficult to remember all of the arguments and definitions associated with a given function. When you start typing the name of a function and hit the Tab key, RStudio will display functions and associated help:
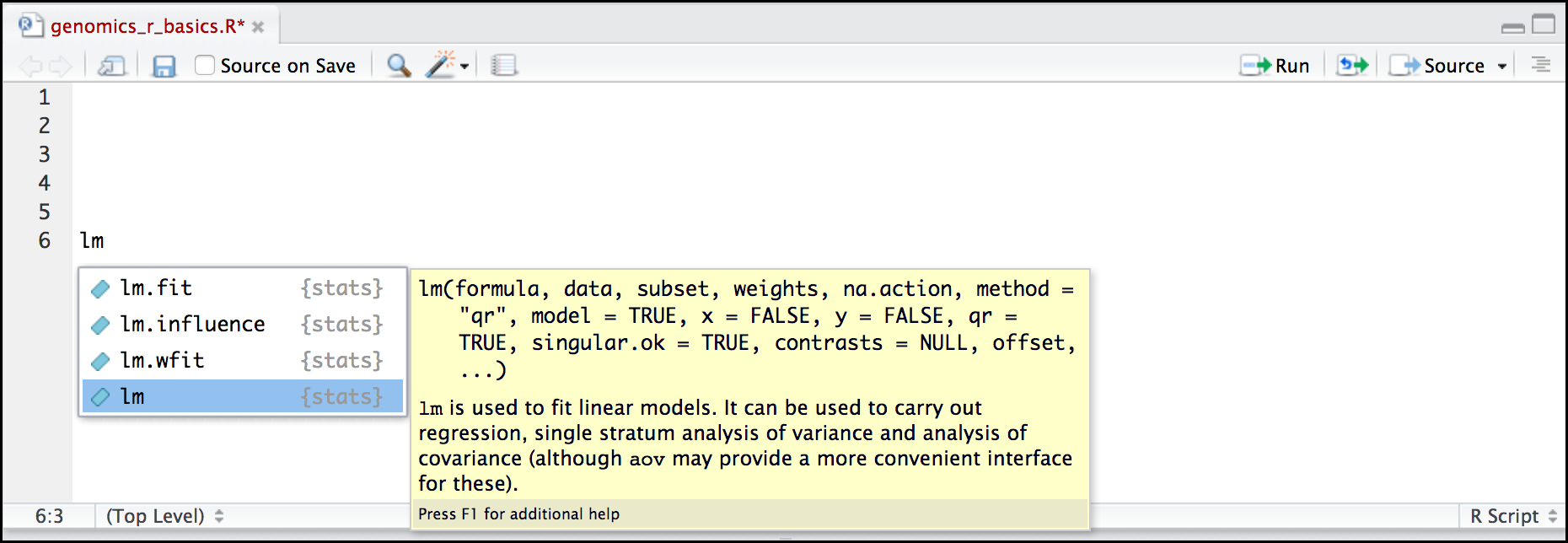
Once you type a function, hitting the Tab inside the parentheses will show you the function’s arguments and provide additional help for each of these arguments.
Content from R Basics
Last updated on 2023-08-15 | Edit this page
Estimated time 80 minutes
Overview
Questions
- What will these lessons not cover?
- What are the basic features of the R language?
- What are the most common objects in R?
Objectives
- Be able to create the most common R objects including vectors
- Understand that vectors have modes, which correspond to the type of data they contain
- Be able to use arithmetic operators on R objects
- Be able to retrieve (subset), name, or replace, values from a vector
- Be able to use logical operators in a subsetting operation
“The fantastic world of R awaits you” OR “Nobody wants to learn how to use R”
Before we begin this lesson, we want you to be clear on the goal of the workshop and these lessons. We believe that every learner can achieve competency with R. You have reached competency when you find that you are able to use R to handle common analysis challenges in a reasonable amount of time (which includes time needed to look at learning materials, search for answers online, and ask colleagues for help). As you spend more time using R (there is no substitute for regular use and practice) you will find yourself gaining competency and even expertise. The more familiar you get, the more complex the analyses you will be able to carry out, with less frustration, and in less time - the fantastic world of R awaits you!
What these lessons will not teach you
Nobody wants to learn how to use R. People want to learn how to use R to analyze their own research questions! Ok, maybe some folks learn R for R’s sake, but these lessons assume that you want to start analyzing genomic data as soon as possible. Given this, there are many valuable pieces of information about R that we simply won’t have time to cover. Hopefully, we will clear the hurdle of giving you just enough knowledge to be dangerous, which can be a high bar in R! We suggest you look into the additional learning materials in the tip box below.
Here are some R skills we will not cover in these lessons
- How to create and work with R matrices
- How to create and work with loops and conditional statements, and the “apply” family of functions (which are super useful, read more here)
- How to do basic string manipulations (e.g. finding patterns in text using grep, replacing text)
- How to plot using the default R graphic tools (we will
cover plot creation, but will do so using the popular plotting package
ggplot2) - How to use advanced R statistical functions
Tip: Where to learn more
The following are good resources for learning more about R. Some of them can be quite technical, but if you are a regular R user you may ultimately need this technical knowledge.
- R for Beginners: By Emmanuel Paradis and a great starting point
- The R Manuals: Maintained by the R project
- R contributed documentation: Also linked to the R project; importantly there are materials available in several languages
- R for Data Science: A wonderful collection by noted R educators and developers Garrett Grolemund and Hadley Wickham
- Practical Data Science for Stats: Not exclusively about R usage, but a nice collection of pre-prints on data science and applications for R
- Programming in R Software Carpentry lesson: There are several Software Carpentry lessons in R to choose from
Creating objects in R
What might be called a variable in many languages is called an object in R.
To create an object you need:
- a name (e.g. ‘a’)
- a value (e.g. ‘1’)
- the assignment operator (‘<-’)
In your script, “genomics_r_basics.R”, using the R assignment operator ‘<-’, assign ‘1’ to the object ‘a’ as shown. Remember to leave a comment in the line above (using the ‘#’) to explain what you are doing:
R
# this line creates the object 'a' and assigns it the value '1'
a <- 1
Next, run this line of code in your script. You can run a line of code by hitting the Run button that is just above the first line of your script in the header of the Source pane or you can use the appropriate shortcut:
- Windows execution shortcut: Ctrl+Enter
- Mac execution shortcut: Cmd(⌘)+Enter
To run multiple lines of code, you can highlight all the line you wish to run and then hit Run or use the shortcut key combo listed above.
In the RStudio ‘Console’ you should see:
OUTPUT
a <- 1
>The ‘Console’ will display lines of code run from a script and any outputs or status/warning/error messages (usually in red).
In the ‘Environment’ window you will also get a table:
| Values | |
|---|---|
| a | 1 |
The ‘Environment’ window allows you to keep track of the objects you have created in R.
Exercise: Create some objects in R
Create the following objects; give each object an appropriate name (your best guess at what name to use is fine):
- Create an object that has the value of number of pairs of human chromosomes
- Create an object that has a value of your favorite gene name
- Create an object that has this URL as its value: “ftp://ftp.ensemblgenomes.org/pub/bacteria/release-39/fasta/bacteria_5_collection/escherichia_coli_b_str_rel606/”
- Create an object that has the value of the number of chromosomes in a diploid human cell
Here as some possible answers to the challenge:
R
human_chr_number <- 23
gene_name <- 'pten'
ensemble_url <- 'ftp://ftp.ensemblgenomes.org/pub/bacteria/release-39/fasta/bacteria_5_collection/escherichia_coli_b_str_rel606/'
human_diploid_chr_num <- 2 * human_chr_number
Naming objects in R
Here are some important details about naming objects in R.
-
Avoid spaces and special characters: Object names
cannot contain spaces or the minus sign (
-). You can use ‘_’ to make names more readable. You should avoid using special characters in your object name (e.g. ! @ # . , etc.). Also, object names cannot begin with a number. - Use short, easy-to-understand names: You should avoid naming your objects using single letters (e.g. ‘n’, ‘p’, etc.). This is mostly to encourage you to use names that would make sense to anyone reading your code (a colleague, or even yourself a year from now). Also, avoiding excessively long names will make your code more readable.
- Avoid commonly used names: There are several names that may already have a definition in the R language (e.g. ‘mean’, ‘min’, ‘max’). One clue that a name already has meaning is that if you start typing a name in RStudio and it gets a colored highlight or RStudio gives you a suggested autocompletion you have chosen a name that has a reserved meaning.
-
Use the recommended assignment operator: In R, we
use ‘<-’ as the preferred assignment operator. ‘=’ works too, but is
most commonly used in passing arguments to functions (more on functions
later). There is a shortcut for the R assignment operator:
- Windows execution shortcut: Alt+-
- Mac execution shortcut: Option+-
There are a few more suggestions about naming and style you may want to learn more about as you write more R code. There are several “style guides” that have advice, and one to start with is the tidyverse R style guide.
Tip: Pay attention to warnings in the script console
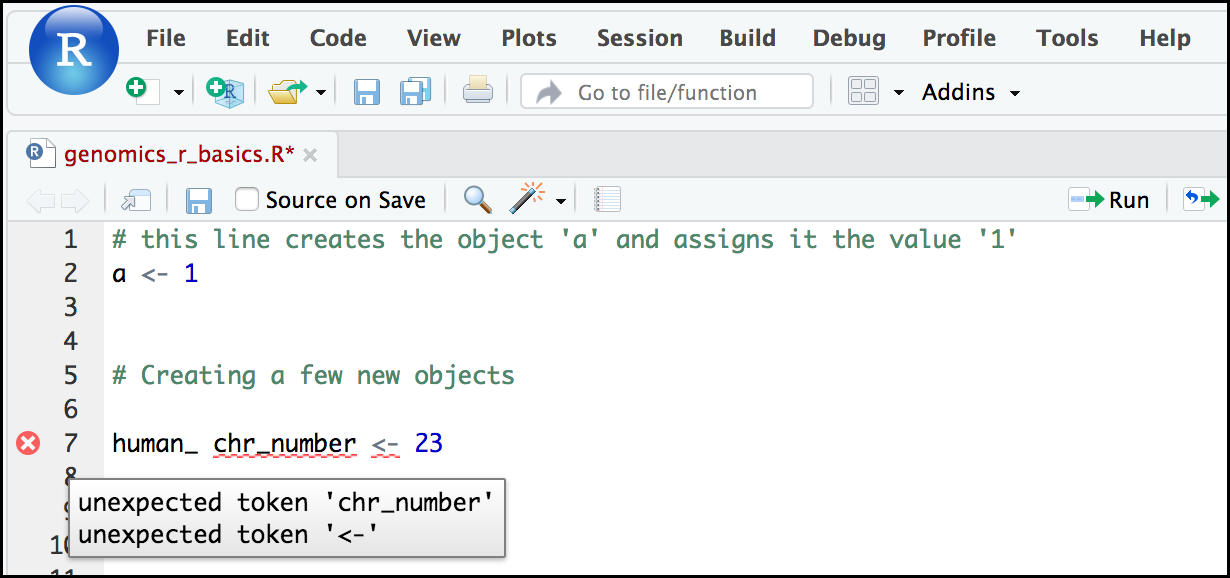
If you enter a line of code in your script that contains an error, RStudio may give you an error message and underline this mistake. Sometimes these messages are easy to understand, but often the messages may need some figuring out. Paying attention to these warnings will help you avoid mistakes. In the example below, our object name has a space, which is not allowed in R. The error message does not say this directly, but R is “not sure” about how to assign the name to “human_ chr_number” when the object name we want is “human_chr_number”.
Reassigning object names or deleting objects
Once an object has a value, you can change that value by overwriting it. R will not give you a warning or error if you overwriting an object, which may or may not be a good thing depending on how you look at it.
R
# gene_name has the value 'pten' or whatever value you used in the challenge.
# We will now assign the new value 'tp53'
gene_name <- 'tp53'
You can also remove an object from R’s memory entirely. The
rm() function will delete the object.
R
# delete the object 'gene_name'
rm(gene_name)
If you run a line of code that has only an object name, R will normally display the contents of that object. In this case, we are told the object no longer exists.
ERROR
Error: object 'gene_name' not foundUnderstanding object data types (modes)
In R, every object has two properties:
- Length: How many distinct values are held in that object
- Mode: What is the classification (type) of that object.
We will get to the “length” property later in the lesson. The “mode” property corresponds to the type of data an object represents. The most common modes you will encounter in R are:
| Mode (abbreviation) | Type of data |
|---|---|
| Numeric (num) | Numbers such floating point/decimals (1.0, 0.5, 3.14), there are also more specific numeric types (dbl - Double, int - Integer). These differences are not relevant for most beginners and pertain to how these values are stored in memory |
| Character (chr) | A sequence of letters/numbers in single ’’ or double ” ” quotes |
| Logical | Boolean values - TRUE or FALSE |
There are a few other modes (i.e. “complex”, “raw” etc.) but these are the three we will work with in this lesson.
Data types are familiar in many programming languages, but also in natural language where we refer to them as the parts of speech, e.g. nouns, verbs, adverbs, etc. Once you know if a word - perhaps an unfamiliar one - is a noun, you can probably guess you can count it and make it plural if there is more than one (e.g. 1 Tuatara, or 2 Tuataras). If something is a adjective, you can usually change it into an adverb by adding “-ly” (e.g. jejune vs. jejunely). Depending on the context, you may need to decide if a word is in one category or another (e.g “cut” may be a noun when it’s on your finger, or a verb when you are preparing vegetables). These concepts have important analogies when working with R objects.
Exercise: Create objects and check their modes
Create the following objects in R, then use the mode()
function to verify their modes. Try to guess what the mode will be
before you look at the solution
chromosome_name <- 'chr02'od_600_value <- 0.47chr_position <- '1001701'spock <- TRUEpilot <- Earhart
ERROR
Error in eval(expr, envir, enclos): object 'Earhart' not foundR
mode(chromosome_name)
OUTPUT
[1] "character"R
mode(od_600_value)
OUTPUT
[1] "numeric"R
mode(chr_position)
OUTPUT
[1] "character"R
mode(spock)
OUTPUT
[1] "logical"R
mode(pilot)
ERROR
Error in eval(expr, envir, enclos): object 'pilot' not foundNotice from the solution that even if a series of numbers is given as
a value R will consider them to be in the “character” mode if they are
enclosed as single or double quotes. Also, notice that you cannot take a
string of alphanumeric characters (e.g. Earhart) and assign as a value
for an object. In this case, R looks for an object named
Earhart but since there is no object, no assignment can be
made. If Earhart did exist, then the mode of
pilot would be whatever the mode of Earhart
was originally. If we want to create an object called pilot
that was the name “Earhart”, we need to enclose
Earhart in quotation marks.
R
pilot <- "Earhart"
mode(pilot)
OUTPUT
[1] "character"Mathematical and functional operations on objects
Once an object exists (which by definition also means it has a mode), R can appropriately manipulate that object. For example, objects of the numeric modes can be added, multiplied, divided, etc. R provides several mathematical (arithmetic) operators including:
| Operator | Description |
|---|---|
| + | addition |
| - | subtraction |
| * | multiplication |
| / | division |
| ^ or ** | exponentiation |
| a%/%b | integer division (division where the remainder is discarded) |
| a%%b | modulus (returns the remainder after division) |
These can be used with literal numbers:
R
(1 + (5 ** 0.5))/2
OUTPUT
[1] 1.618034and importantly, can be used on any object that evaluates to (i.e. interpreted by R) a numeric object:
R
# multiply the object 'human_chr_number' by 2
human_chr_number * 2
OUTPUT
[1] 46Exercise: Compute the golden ratio
One approximation of the golden ratio (φ) can be found by taking the
sum of 1 and the square root of 5, and dividing by 2 as in the example
above. Compute the golden ratio to 3 digits of precision using the
sqrt() and round() functions. Hint: remember
the round() function can take 2 arguments.
R
round((1 + sqrt(5))/2, digits = 3)
OUTPUT
[1] 1.618Notice that you can place one function inside of another.
Vectors
Vectors are probably the most used commonly used object type in R.
A vector is a collection of values that are all of the same type
(numbers, characters, etc.). One of the most common ways to
create a vector is to use the c() function - the
“concatenate” or “combine” function. Inside the function you may enter
one or more values; for multiple values, separate each value with a
comma:
R
# Create the SNP gene name vector
snp_genes <- c("OXTR", "ACTN3", "AR", "OPRM1")
Vectors always have a mode and a
length. You can check these with the
mode() and length() functions respectively.
Another useful function that gives both of these pieces of information
is the str() (structure) function.
R
# Check the mode, length, and structure of 'snp_genes'
mode(snp_genes)
OUTPUT
[1] "character"R
length(snp_genes)
OUTPUT
[1] 4R
str(snp_genes)
OUTPUT
chr [1:4] "OXTR" "ACTN3" "AR" "OPRM1"Vectors are quite important in R. Another data type that we will work with later in this lesson, data frames, are collections of vectors. What we learn here about vectors will pay off even more when we start working with data frames.
Creating and subsetting vectors
Let’s create a few more vectors to play around with:
R
# Some interesting human SNPs
# while accuracy is important, typos in the data won't hurt you here
snps <- c("rs53576", "rs1815739", "rs6152", "rs1799971")
snp_chromosomes <- c("3", "11", "X", "6")
snp_positions <- c(8762685, 66560624, 67545785, 154039662)
Once we have vectors, one thing we may want to do is specifically retrieve one or more values from our vector. To do so, we use bracket notation. We type the name of the vector followed by square brackets. In those square brackets we place the index (e.g. a number) in that bracket as follows:
R
# get the 3rd value in the snp vector
snp[3]
ERROR
Error in eval(expr, envir, enclos): object 'snp' not foundIn R, every item your vector is indexed, starting from the first item (1) through to the final number of items in your vector. You can also retrieve a range of numbers:
R
# get the 1st through 3rd value in the snp vector
snp[1:3]
ERROR
Error in eval(expr, envir, enclos): object 'snp' not foundIf you want to retrieve several (but not necessarily sequential) items from a vector, you pass a vector of indices; a vector that has the numbered positions you wish to retrieve.
R
# get the 1st, 3rd, and 4th value in the snp vector
snp[c(1, 3, 4)]
ERROR
Error in eval(expr, envir, enclos): object 'snp' not foundThere are additional (and perhaps less commonly used) ways of subsetting a vector (see these examples). Also, several of these subsetting expressions can be combined:
R
# get the 1st through the 3rd value, and 4th value in the snp vector
# yes, this is a little silly in a vector of only 4 values.
snp[c(1:3,4)]
ERROR
Error in eval(expr, envir, enclos): object 'snp' not foundAdding to, removing, or replacing values in existing vectors
Once you have an existing vector, you may want to add a new item to
it. To do so, you can use the c() function again to add
your new value:
R
# add the gene "CYP1A1" and "APOA5" to our list of snp genes
# this overwrites our existing vector
snp_genes <- c(snp_genes, "CYP1A1", "APOA5")
We can verify that “snp_genes” contains the new gene entry
R
snp_genes
OUTPUT
[1] "OXTR" "ACTN3" "AR" "OPRM1" "CYP1A1" "APOA5" Using a negative index will return a version of a vector with that index’s value removed:
R
snp_genes[-6]
OUTPUT
[1] "OXTR" "ACTN3" "AR" "OPRM1" "CYP1A1"We can remove that value from our vector by overwriting it with this expression:
R
snp_genes <- snp_genes[-6]
snp_genes
OUTPUT
[1] "OXTR" "ACTN3" "AR" "OPRM1" "CYP1A1"We can also explicitly rename or add a value to our index using double bracket notation:
R
snp_genes[6]<- "APOA5"
snp_genes
OUTPUT
[1] "OXTR" "ACTN3" "AR" "OPRM1" "CYP1A1" "APOA5" Exercise: Examining and subsetting vectors
Answer the following questions to test your knowledge of vectors
Which of the following are true of vectors in R? A) All vectors have
a mode or a length
B) All vectors have a mode and a length
C) Vectors may have different lengths
D) Items within a vector may be of different modes
E) You can use the c() to add one or more items to an
existing vector
F) You can use the c() to add a vector to an exiting
vector
- False - Vectors have both of these properties
- True
- True
- False - Vectors have only one mode (e.g. numeric, character); all
items in
a vector must be of this mode. - True
- True
Logical Subsetting
There is one last set of cool subsetting capabilities we want to introduce. It is possible within R to retrieve items in a vector based on a logical evaluation or numerical comparison. For example, let’s say we wanted get all of the SNPs in our vector of SNP positions that were greater than 100,000,000. We could index using the ‘>’ (greater than) logical operator:
R
snp_positions[snp_positions > 100000000]
OUTPUT
[1] 154039662In the square brackets you place the name of the vector followed by the comparison operator and (in this case) a numeric value. Some of the most common logical operators you will use in R are:
| Operator | Description |
|---|---|
| < | less than |
| <= | less than or equal to |
| > | greater than |
| >= | greater than or equal to |
| == | exactly equal to |
| != | not equal to |
| !x | not x |
| a | b |
| a & b | a and b |
The magic of programming
The reason why the expression
snp_positions[snp_positions > 100000000] works can be
better understood if you examine what the expression “snp_positions >
100000000” evaluates to:
R
snp_positions > 100000000
OUTPUT
[1] FALSE FALSE FALSE TRUEThe output above is a logical vector, the 4th element of which is TRUE. When you pass a logical vector as an index, R will return the true values:
R
snp_positions[c(FALSE, FALSE, FALSE, TRUE)]
OUTPUT
[1] 154039662If you have never coded before, this type of situation starts to
expose the “magic” of programming. We mentioned before that in the
bracket notation you take your named vector followed by brackets which
contain an index: named_vector[index]. The “magic” is
that the index needs to evaluate to a number. So, even if it
does not appear to be an integer (e.g. 1, 2, 3), as long as R can
evaluate it, we will get a result. That our expression
snp_positions[snp_positions > 100000000] evaluates to a
number can be seen in the following situation. If you wanted to know
which index (1, 2, 3, or 4) in our vector of SNP
positions was the one that was greater than 100,000,000?
We can use the which() function to return the indices of
any item that evaluates as TRUE in our comparison:
R
which(snp_positions > 100000000)
OUTPUT
[1] 4Why this is important
Often in programming we will not know what inputs and values will be used when our code is executed. Rather than put in a pre-determined value (e.g 100000000) we can use an object that can take on whatever value we need. So for example:
R
snp_marker_cutoff <- 100000000
snp_positions[snp_positions > snp_marker_cutoff]
OUTPUT
[1] 154039662Ultimately, it’s putting together flexible, reusable code like this that gets at the “magic” of programming!
A few final vector tricks
Finally, there are a few other common retrieve or replace operations
you may want to know about. First, you can check to see if any of the
values of your vector are missing (i.e. are NA, that stands
for not avaliable). Missing data will get a more detailed
treatment later, but the is.NA() function will return a
logical vector, with TRUE for any NA value:
R
# current value of 'snp_genes':
# chr [1:7] "OXTR" "ACTN3" "AR" "OPRM1" "CYP1A1" NA "APOA5"
is.na(snp_genes)
OUTPUT
[1] FALSE FALSE FALSE FALSE FALSE FALSESometimes, you may wish to find out if a specific value (or several
values) is present a vector. You can do this using the comparison
operator %in%, which will return TRUE for any value in your
collection that is in the vector you are searching:
R
# current value of 'snp_genes':
# chr [1:7] "OXTR" "ACTN3" "AR" "OPRM1" "CYP1A1" NA "APOA5"
# test to see if "ACTN3" or "APO5A" is in the snp_genes vector
# if you are looking for more than one value, you must pass this as a vector
c("ACTN3","APOA5") %in% snp_genes
OUTPUT
[1] TRUE TRUER
mode(snps)
OUTPUT
[1] "character"R
mode(snp_chromosomes)
OUTPUT
[1] "character"R
mode(snp_positions)
OUTPUT
[1] "numeric"R
snps <- c(snps, "rs662799")
snps
OUTPUT
[1] "rs53576" "rs1815739" "rs6152" "rs1799971" "rs662799" R
snp_chromosomes <- c(snp_chromosomes, "11") # did you use quotes?
snp_chromosomes
OUTPUT
[1] "3" "11" "X" "6" "11"R
snp_positions <- c(snp_positions, 116792991)
snp_positions
OUTPUT
[1] 8762685 66560624 67545785 154039662 116792991Review Exercise 3
Make the following change to the snp_genes vector:
Hint: Your vector should look like this in ‘Environment’:
chr [1:7] "OXTR" "ACTN3" "AR" "OPRM1" "CYP1A1" NA "APOA5".
If not recreate the vector by running this expression:
snp_genes <- c("OXTR", "ACTN3", "AR", "OPRM1", "CYP1A1", NA, "APOA5")
- Create a new version of
snp_genesthat does not contain CYP1A1 and then
- Add 2 NA values to the end of
snp_genes
R
snp_genes <- snp_genes[-5]
snp_genes <- c(snp_genes, NA, NA)
snp_genes
OUTPUT
[1] "OXTR" "ACTN3" "AR" "OPRM1" "APOA5" NA NA R
combined <- c(snp_genes[1], snps[1], snp_chromosomes[1], snp_positions[1])
combined
OUTPUT
[1] "OXTR" "rs53576" "3" "8762685"R
typeof(combined)
OUTPUT
[1] "character"Lists
Lists are quite useful in R, but we won’t be using them in the genomics lessons. That said, you may come across lists in the way that some bioinformatics programs may store and/or return data to you. One of the key attributes of a list is that, unlike a vector, a list may contain data of more than one mode. Learn more about creating and using lists using this nice tutorial. In this one example, we will create a named list and show you how to retrieve items from the list.
R
# Create a named list using the 'list' function and our SNP examples
# Note, for easy reading we have placed each item in the list on a separate line
# Nothing special about this, you can do this for any multiline commands
# To run this command, make sure the entire command (all 4 lines) are highlighted
# before running
# Note also, as we are doing all this inside the list() function use of the
# '=' sign is good style
snp_data <- list(genes = snp_genes,
refference_snp = snps,
chromosome = snp_chromosomes,
position = snp_positions)
# Examine the structure of the list
str(snp_data)
OUTPUT
List of 4
$ genes : chr [1:7] "OXTR" "ACTN3" "AR" "OPRM1" ...
$ refference_snp: chr [1:5] "rs53576" "rs1815739" "rs6152" "rs1799971" ...
$ chromosome : chr [1:5] "3" "11" "X" "6" ...
$ position : num [1:5] 8.76e+06 6.66e+07 6.75e+07 1.54e+08 1.17e+08To get all the values for the position object in the
list, we use the $ notation:
R
# return all the values of position object
snp_data$position
OUTPUT
[1] 8762685 66560624 67545785 154039662 116792991To get the first value in the position object, use the
[] notation to index:
R
# return first value of the position object
snp_data$position[1]
OUTPUT
[1] 8762685Keypoints
- Effectively using R is a journey of months or years. Still you don’t have to be an expert to use R and you can start using and analyzing your data with with about a day’s worth of training
- It is important to understand how data are organized by R in a given object type and how the mode of that type (e.g. numeric, character, logical, etc.) will determine how R will operate on that data.
- Working with vectors effectively prepares you for understanding how data are organized in R.
Content from Introduction to the example dataset and file type
Last updated on 2023-08-15 | Edit this page
Estimated time 15 minutes
Overview
Questions
- What data are we using in the lesson?
- What are VCF files?
Objectives
- Know what the example dataset represents
- Know the concepts of how VCF files are generated
Preface
The Intro to R and RStudio for Genomics is a part of the Genomics Data Carpentry lessons. In this lesson we will learn the necessary skill sets for R and RStudio and apply them directly to a real next-generation sequencing (NGS) data in the variant calling format (VCF) file type. Previous Genomics Data Carpentry lessons teach learners how to generate a VCF file from FASTQ files downloaded from NCBI Sequence Read Archive (SRA), so we won’t cover that here. Instead, in this episode we will give a brief overview of the data and a what VCF file types are for those who wish to teach the Intro to R and RStudio for Genomics lesson independently of the Genomics Data Carpentry lessons.
This dataset was selected for several reasons, including:
- Simple, but iconic NGS-problem: Examine a population where we want to characterize changes in sequence a priori
- Dataset publicly available - in this case through the NCBI SRA (http://www.ncbi.nlm.nih.gov/sra)
Introduction to the dataset
Microbes are ideal organisms for exploring ‘Long-term Evolution Experiments’ (LTEEs) - thousands of generations can be generated and stored in a way that would be virtually impossible for more complex eukaryotic systems. In Tenaillon et al 2016, 12 populations of Escherichia coli were propagated for more than 50,000 generations in a glucose-limited minimal medium. This medium was supplemented with citrate which E. coli cannot metabolize in the aerobic conditions of the experiment. Sequencing of the populations at regular time points reveals that spontaneous citrate-using mutants (Cit+) appeared in a population of E.coli (designated Ara-3) at around 31,000 generations. It should be noted that spontaneous Cit+ mutants are extraordinarily rare - inability to metabolize citrate is one of the defining characters of the E. coli species. Eventually, Cit+ mutants became the dominant population as the experimental growth medium contained a high concentration of citrate relative to glucose. Around the same time that this mutation emerged, another phenotype become prominent in the Ara-3 population. Many E. coli began to develop excessive numbers of mutations, meaning they became hypermutable.
Strains from generation 0 to generation 50,000 were sequenced, including ones that were both Cit+ and Cit- and hypermutable in later generations.
For the purposes of this workshop we’re going to be working with 3 of the sequence reads from this experiment.
| SRA Run Number | Clone | Generation | Cit | Hypermutable | Read Length | Sequencing Depth |
|---|---|---|---|---|---|---|
| SRR2589044 | REL2181A | 5,000 | Unknown | None | 150 | 60.2 |
| SRR2584863 | REL7179B | 15,000 | Unknown | None | 150 | 88 |
| SRR2584866 | REL11365 | 50,000 | Cit+ | plus | 150 | 138.3 |
We want to be able to look at differences in mutation rates between hypermutable and non-hypermutable strains. We also want to analyze the sequences to figure out what changes occurred in genomes to make the strains Cit+. Ultimately, we will use R to answer the questions:
- How many base pair changes are there between the Cit+ and Cit- strains?
- What are the base pair changes between strains?
How VCF files are generated
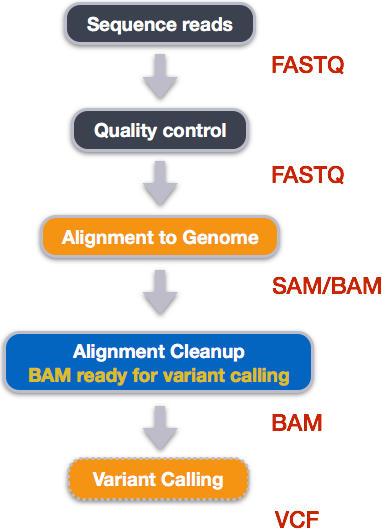
Publicly accessible sequencing files in FASTQ formats can be downloaded from NCBI SRA. However, at FASTQ files contain unaligned sequences of varying quality, and requires clean up and alignment steps for variants to be called from the reference genome.
Five steps are taken to transform FASTQ files to variant calls contained in VCF files and at each step, specialized non-R based bioinformatics tools that are used:
How variant calls are stored in VCF files
VCF files contain variants that were called against a reference genome. These files are slightly more complicated than regular tables you can open using programs like Excel and contain two sections: header and records.
Below you will see the header (which describes the format), the time and date the file was created, the version of bcftools that was used, the command line parameters used, and some additional information:
##fileformat=VCFv4.2
##FILTER=<ID=PASS,Description="All filters passed">
##bcftoolsVersion=1.8+htslib-1.8
##bcftoolsCommand=mpileup -O b -o results/bcf/SRR2584866_raw.bcf -f data/ref_genome/ecoli_rel606.fasta results/bam/SRR2584866.aligned.sorted.bam
##reference=file://data/ref_genome/ecoli_rel606.fasta
##contig=<ID=CP000819.1,length=4629812>
##ALT=<ID=*,Description="Represents allele(s) other than observed.">
##INFO=<ID=INDEL,Number=0,Type=Flag,Description="Indicates that the variant is an INDEL.">
##INFO=<ID=IDV,Number=1,Type=Integer,Description="Maximum number of reads supporting an indel">
##INFO=<ID=IMF,Number=1,Type=Float,Description="Maximum fraction of reads supporting an indel">
##INFO=<ID=DP,Number=1,Type=Integer,Description="Raw read depth">
##INFO=<ID=VDB,Number=1,Type=Float,Description="Variant Distance Bias for filtering splice-site artefacts in RNA-seq data (bigger is better)",Version=
##INFO=<ID=RPB,Number=1,Type=Float,Description="Mann-Whitney U test of Read Position Bias (bigger is better)">
##INFO=<ID=MQB,Number=1,Type=Float,Description="Mann-Whitney U test of Mapping Quality Bias (bigger is better)">
##INFO=<ID=BQB,Number=1,Type=Float,Description="Mann-Whitney U test of Base Quality Bias (bigger is better)">
##INFO=<ID=MQSB,Number=1,Type=Float,Description="Mann-Whitney U test of Mapping Quality vs Strand Bias (bigger is better)">
##INFO=<ID=SGB,Number=1,Type=Float,Description="Segregation based metric.">
##INFO=<ID=MQ0F,Number=1,Type=Float,Description="Fraction of MQ0 reads (smaller is better)">
##FORMAT=<ID=PL,Number=G,Type=Integer,Description="List of Phred-scaled genotype likelihoods">
##FORMAT=<ID=GT,Number=1,Type=String,Description="Genotype">
##INFO=<ID=ICB,Number=1,Type=Float,Description="Inbreeding Coefficient Binomial test (bigger is better)">
##INFO=<ID=HOB,Number=1,Type=Float,Description="Bias in the number of HOMs number (smaller is better)">
##INFO=<ID=AC,Number=A,Type=Integer,Description="Allele count in genotypes for each ALT allele, in the same order as listed">
##INFO=<ID=AN,Number=1,Type=Integer,Description="Total number of alleles in called genotypes">
##INFO=<ID=DP4,Number=4,Type=Integer,Description="Number of high-quality ref-forward , ref-reverse, alt-forward and alt-reverse bases">
##INFO=<ID=MQ,Number=1,Type=Integer,Description="Average mapping quality">
##bcftools_callVersion=1.8+htslib-1.8
##bcftools_callCommand=call --ploidy 1 -m -v -o results/bcf/SRR2584866_variants.vcf results/bcf/SRR2584866_raw.bcf; Date=Tue Oct 9 18:48:10 2018Followed by information on each of the variations observed:
#CHROM POS ID REF ALT QUAL FILTER INFO FORMAT results/bam/SRR2584866.aligned.sorted.bam
CP000819.1 1521 . C T 207 . DP=9;VDB=0.993024;SGB=-0.662043;MQSB=0.974597;MQ0F=0;AC=1;AN=1;DP4=0,0,4,5;MQ=60
CP000819.1 1612 . A G 225 . DP=13;VDB=0.52194;SGB=-0.676189;MQSB=0.950952;MQ0F=0;AC=1;AN=1;DP4=0,0,6,5;MQ=60
CP000819.1 9092 . A G 225 . DP=14;VDB=0.717543;SGB=-0.670168;MQSB=0.916482;MQ0F=0;AC=1;AN=1;DP4=0,0,7,3;MQ=60
CP000819.1 9972 . T G 214 . DP=10;VDB=0.022095;SGB=-0.670168;MQSB=1;MQ0F=0;AC=1;AN=1;DP4=0,0,2,8;MQ=60 GT:PL
CP000819.1 10563 . G A 225 . DP=11;VDB=0.958658;SGB=-0.670168;MQSB=0.952347;MQ0F=0;AC=1;AN=1;DP4=0,0,5,5;MQ=60
CP000819.1 22257 . C T 127 . DP=5;VDB=0.0765947;SGB=-0.590765;MQSB=1;MQ0F=0;AC=1;AN=1;DP4=0,0,2,3;MQ=60 GT:PL
CP000819.1 38971 . A G 225 . DP=14;VDB=0.872139;SGB=-0.680642;MQSB=1;MQ0F=0;AC=1;AN=1;DP4=0,0,4,8;MQ=60 GT:PL
CP000819.1 42306 . A G 225 . DP=15;VDB=0.969686;SGB=-0.686358;MQSB=1;MQ0F=0;AC=1;AN=1;DP4=0,0,5,9;MQ=60 GT:PL
CP000819.1 45277 . A G 225 . DP=15;VDB=0.470998;SGB=-0.680642;MQSB=0.95494;MQ0F=0;AC=1;AN=1;DP4=0,0,7,5;MQ=60
CP000819.1 56613 . C G 183 . DP=12;VDB=0.879703;SGB=-0.676189;MQSB=1;MQ0F=0;AC=1;AN=1;DP4=0,0,8,3;MQ=60 GT:PL
CP000819.1 62118 . A G 225 . DP=19;VDB=0.414981;SGB=-0.691153;MQSB=0.906029;MQ0F=0;AC=1;AN=1;DP4=0,0,8,10;MQ=59
CP000819.1 64042 . G A 225 . DP=18;VDB=0.451328;SGB=-0.689466;MQSB=1;MQ0F=0;AC=1;AN=1;DP4=0,0,7,9;MQ=60 GT:PLThe first few columns represent the information we have about a predicted variation.
| column | info |
|---|---|
| CHROM | contig location where the variation occurs |
| POS | position within the contig where the variation occurs |
| ID | a . until we add annotation information |
| REF | reference genotype (forward strand) |
| ALT | sample genotype (forward strand) |
| QUAL | Phred-scaled probability that the observed variant exists at this site (higher is better) |
| FILTER | a . if no quality filters have been applied, PASS if a
filter is passed, or the name of the filters this variant failed |
In an ideal world, the information in the QUAL column
would be all we needed to filter out bad variant calls. However, in
reality we need to filter on multiple other metrics.
The last two columns contain the genotypes and can be tricky to decode.
| column | info |
|---|---|
| FORMAT | lists in order the metrics presented in the final column |
| results | lists the values associated with those metrics in order |
For our file, the metrics presented are GT:PL:GQ.
| metric | definition |
|---|---|
| AD, DP | the depth per allele by sample and coverage |
| GT | the genotype for the sample at this loci. For a diploid organism, the GT field indicates the two alleles carried by the sample, encoded by a 0 for the REF allele, 1 for the first ALT allele, 2 for the second ALT allele, etc. A 0/0 means homozygous reference, 0/1 is heterozygous, and 1/1 is homozygous for the alternate allele. |
| PL | the likelihoods of the given genotypes |
| GQ | the Phred-scaled confidence for the genotype |
For more information on VCF files visit The Broad Institute’s VCF guide.
References
Tenaillon O, Barrick JE, Ribeck N, Deatherage DE, Blanchard JL, Dasgupta A, Wu GC, Wielgoss S, Cruveiller S, Médigue C, Schneider D, Lenski RE. Tempo and mode of genome evolution in a 50,000-generation experiment (2016) Nature. 536(7615): 165–170. Paper, Supplemental materials Data on NCBI SRA: https://trace.ncbi.nlm.nih.gov/Traces/sra/?study=SRP064605 Data on EMBL-EBI ENA: https://www.ebi.ac.uk/ena/data/view/PRJNA295606
This episode was adapted from the Data Carpentry Genomic lessons:
Content from R Basics continued - factors and data frames
Last updated on 2023-08-15 | Edit this page
Estimated time 90 minutes
Overview
Questions
- How do I get started with tabular data (e.g. spreadsheets) in R?
- What are some best practices for reading data into R?
- How do I save tabular data generated in R?
Objectives
- Explain the basic principle of tidy datasets
- Be able to load a tabular dataset using base R functions
- Be able to determine the structure of a data frame including its dimensions and the datatypes of variables
- Be able to subset/retrieve values from a data frame
- Understand how R may coerce data into different modes
- Be able to change the mode of an object
- Understand that R uses factors to store and manipulate categorical data
- Be able to manipulate a factor, including subsetting and reordering
- Be able to apply an arithmetic function to a data frame
- Be able to coerce the class of an object (including variables in a data frame)
- Be able to import data from Excel
- Be able to save a data frame as a delimited file
Working with spreadsheets (tabular data)
A substantial amount of the data we work with in genomics will be tabular data, this is data arranged in rows and columns - also known as spreadsheets. We could write a whole lesson on how to work with spreadsheets effectively (actually we did). For our purposes, we want to remind you of a few principles before we work with our first set of example data:
1) Keep raw data separate from analyzed data
This is principle number one because if you can’t tell which files are the original raw data, you risk making some serious mistakes (e.g. drawing conclusion from data which have been manipulated in some unknown way).
2) Keep spreadsheet data Tidy
The simplest principle of Tidy data is that we have one row in our spreadsheet for each observation or sample, and one column for every variable that we measure or report on. As simple as this sounds, it’s very easily violated. Most data scientists agree that significant amounts of their time is spent tidying data for analysis. Read more about data organization in our lesson and in this paper.
3) Trust but verify
Finally, while you don’t need to be paranoid about data, you should have a plan for how you will prepare it for analysis. This a focus of this lesson. You probably already have a lot of intuition, expectations, assumptions about your data - the range of values you expect, how many values should have been recorded, etc. Of course, as the data get larger our human ability to keep track will start to fail (and yes, it can fail for small data sets too). R will help you to examine your data so that you can have greater confidence in your analysis, and its reproducibility.
Tip: Keeping you raw data separate
When you work with data in R, you are not changing the original file
you loaded that data from. This is different than (for example) working
with a spreadsheet program where changing the value of the cell leaves
you one “save”-click away from overwriting the original file. You have
to purposely use a writing function (e.g. write.csv()) to
save data loaded into R. In that case, be sure to save the manipulated
data into a new file. More on this later in the lesson.
Importing tabular data into R
There are several ways to import data into R. For our purpose here,
we will focus on using the tools every R installation comes with (so
called “base” R) to import a comma-delimited file containing the results
of our variant calling workflow. We will need to load the sheet using a
function called read.csv().
Exercise: Review the arguments of the
read.csv() function
Before using the read.csv() function, use R’s
help feature to answer the following questions.
Hint: Entering ‘?’ before the function name and then running that line will bring up the help documentation. Also, when reading this particular help be careful to pay attention to the ‘read.csv’ expression under the ‘Usage’ heading. Other answers will be in the ‘Arguments’ heading.
What is the default parameter for ‘header’ in the
read.csv()function?What argument would you have to change to read a file that was delimited by semicolons (;) rather than commas?
What argument would you have to change to read file in which numbers used commas for decimal separation (i.e. 1,00)?
What argument would you have to change to read in only the first 10,000 rows of a very large file?
The
read.csv()function has the argument ‘header’ set to TRUE by default, this means the function always assumes the first row is header information, (i.e. column names)The
read.csv()function has the argument ‘sep’ set to “,”. This means the function assumes commas are used as delimiters, as you would expect. Changing this parameter (e.g.sep=";") would now interpret semicolons as delimiters.Although it is not listed in the
read.csv()usage,read.csv()is a “version” of the functionread.table()and accepts all its arguments. If you setdec=","you could change the decimal operator. We’d probably assume the delimiter is some other character.You can set
nrowto a numeric value (e.g.nrow=10000) to choose how many rows of a file you read in. This may be useful for very large files where not all the data is needed to test some data cleaning steps you are applying.
Hopefully, this exercise gets you thinking about using the provided help documentation in R. There are many arguments that exist, but which we wont have time to cover. Look here to get familiar with functions you use frequently, you may be surprised at what you find they can do.
Now, let’s read in the file combined_tidy_vcf.csv which
will be located in /home/dcuser/r_data/. Call this data
variants. The first argument to pass to our
read.csv() function is the file path for our data. The file
path must be in quotes and now is a good time to remember to use tab
autocompletion. If you use tab autocompletion you avoid typos
and errors in file paths. Use it!
R
## read in a CSV file and save it as 'variants'
variants <- read.csv("/home/dcuser/r_data/combined_tidy_vcf.csv")
One of the first things you should notice is that in the Environment
window, you have the variants object, listed as 801 obs.
(observations/rows) of 29 variables (columns). Double-clicking on the
name of the object will open a view of the data in a new tab.
Summarizing, subsetting, and determining the structure of a data frame.
A data frame is the standard way in R to store tabular data. A data fame could also be thought of as a collection of vectors, all of which have the same length. Using only two functions, we can learn a lot about out data frame including some summary statistics as well as well as the “structure” of the data frame. Let’s examine what each of these functions can tell us:
R
## get summary statistics on a data frame
summary(variants)
OUTPUT
sample_id CHROM POS ID
Length:801 Length:801 Min. : 1521 Mode:logical
Class :character Class :character 1st Qu.:1115970 NA's:801
Mode :character Mode :character Median :2290361
Mean :2243682
3rd Qu.:3317082
Max. :4629225
REF ALT QUAL FILTER
Length:801 Length:801 Min. : 4.385 Mode:logical
Class :character Class :character 1st Qu.:139.000 NA's:801
Mode :character Mode :character Median :195.000
Mean :172.276
3rd Qu.:225.000
Max. :228.000
INDEL IDV IMF DP
Mode :logical Min. : 2.000 Min. :0.5714 Min. : 2.00
FALSE:700 1st Qu.: 7.000 1st Qu.:0.8824 1st Qu.: 7.00
TRUE :101 Median : 9.000 Median :1.0000 Median :10.00
Mean : 9.396 Mean :0.9219 Mean :10.57
3rd Qu.:11.000 3rd Qu.:1.0000 3rd Qu.:13.00
Max. :20.000 Max. :1.0000 Max. :79.00
NA's :700 NA's :700
VDB RPB MQB BQB
Min. :0.0005387 Min. :0.0000 Min. :0.0000 Min. :0.1153
1st Qu.:0.2180410 1st Qu.:0.3776 1st Qu.:0.1070 1st Qu.:0.6963
Median :0.4827410 Median :0.8663 Median :0.2872 Median :0.8615
Mean :0.4926291 Mean :0.6970 Mean :0.5330 Mean :0.7784
3rd Qu.:0.7598940 3rd Qu.:1.0000 3rd Qu.:1.0000 3rd Qu.:1.0000
Max. :0.9997130 Max. :1.0000 Max. :1.0000 Max. :1.0000
NA's :773 NA's :773 NA's :773
MQSB SGB MQ0F ICB
Min. :0.01348 Min. :-0.6931 Min. :0.00000 Mode:logical
1st Qu.:0.95494 1st Qu.:-0.6762 1st Qu.:0.00000 NA's:801
Median :1.00000 Median :-0.6620 Median :0.00000
Mean :0.96428 Mean :-0.6444 Mean :0.01127
3rd Qu.:1.00000 3rd Qu.:-0.6364 3rd Qu.:0.00000
Max. :1.01283 Max. :-0.4536 Max. :0.66667
NA's :48
HOB AC AN DP4 MQ
Mode:logical Min. :1 Min. :1 Length:801 Min. :10.00
NA's:801 1st Qu.:1 1st Qu.:1 Class :character 1st Qu.:60.00
Median :1 Median :1 Mode :character Median :60.00
Mean :1 Mean :1 Mean :58.19
3rd Qu.:1 3rd Qu.:1 3rd Qu.:60.00
Max. :1 Max. :1 Max. :60.00
Indiv gt_PL gt_GT gt_GT_alleles
Length:801 Length:801 Min. :1 Length:801
Class :character Class :character 1st Qu.:1 Class :character
Mode :character Mode :character Median :1 Mode :character
Mean :1
3rd Qu.:1
Max. :1
Our data frame had 29 variables, so we get 29 fields that summarize
the data. The QUAL, IMF, and VDB
variables (and several others) are numerical data and so you get summary
statistics on the min and max values for these columns, as well as mean,
median, and interquartile ranges. Many of the other variables
(e.g. sample_id) are treated as characters data (more on
this in a bit).
There is a lot to work with, so we will subset the first three
columns into a new data frame using the data.frame()
function.
R
## put the first three columns of variants into a new data frame called subset
subset<-data.frame(variants[,c(1:3,6)])
Now, let’s use the str() (structure) function to look a
little more closely at how data frames work:
R
## get the structure of a data frame
str(subset)
OUTPUT
'data.frame': 801 obs. of 4 variables:
$ sample_id: chr "SRR2584863" "SRR2584863" "SRR2584863" "SRR2584863" ...
$ CHROM : chr "CP000819.1" "CP000819.1" "CP000819.1" "CP000819.1" ...
$ POS : int 9972 263235 281923 433359 473901 648692 1331794 1733343 2103887 2333538 ...
$ ALT : chr "G" "T" "T" "CTTTTTTTT" ...Ok, thats a lot up unpack! Some things to notice.
- the object type
data.frameis displayed in the first row along with its dimensions, in this case 801 observations (rows) and 4 variables (columns) - Each variable (column) has a name (e.g.
sample_id). This is followed by the object mode (e.g. chr, int, etc.). Notice that before each variable name there is a$- this will be important later.
Introducing Factors
Factors are the final major data structure we will introduce in our R genomics lessons. Factors can be thought of as vectors which are specialized for categorical data. Given R’s specialization for statistics, this make sense since categorial and continuous variables are usually treated differently. Sometimes you may want to have data treated as a factor, but in other cases, this may be undesirable.
Let’s see the value of treating some of which are categorical in nature as factors. Let’s take a look at just the alternate alleles
R
## extract the "ALT" column to a new object
alt_alleles <- subset$ALT
Let’s look at the first few items in our factor using
head():
R
head(alt_alleles)
OUTPUT
[1] "G" "T" "T" "CTTTTTTTT" "CCGCGC" "T" There are 801 alleles (one for each row). To simplify, lets look at just the single-nuleotide alleles (SNPs). We can use some of the vector indexing skills from the last episode.
R
snps <- c(alt_alleles[alt_alleles=="A"],
alt_alleles[alt_alleles=="T"],
alt_alleles[alt_alleles=="G"],
alt_alleles[alt_alleles=="C"])
This leaves us with a vector of the 701 alternative alleles which were single nucleotides. Right now, they are being treated a characters, but we could treat them as categories of SNP. Doing this will enable some nice features. For example, we can try to generate a plot of this character vector as it is right now:
R
plot(snps)
WARNING
Warning in xy.coords(x, y, xlabel, ylabel, log): NAs introduced by coercionWARNING
Warning in min(x): no non-missing arguments to min; returning InfWARNING
Warning in max(x): no non-missing arguments to max; returning -InfERROR
Error in plot.window(...): need finite 'ylim' valuesWhoops! Though the plot() function will do its best to
give us a quick plot, it is unable to do so here. One way to fix this it
to tell R to treat the SNPs as categories (i.e. a factor vector); we
will create a new object to avoid confusion using the
factor() function:
R
factor_snps <- factor(snps)
Let’s learn a little more about this new type of vector:
R
str(factor_snps)
OUTPUT
Factor w/ 4 levels "A","C","G","T": 1 1 1 1 1 1 1 1 1 1 ...What we get back are the categories (“A”,“C”,“G”,“T”) in our factor; these are called “Levels”. Levels are the different categories contained in a factor. By default, R will organize the levels in a factor in alphabetical order. So the first level in this factor is “A”.
For the sake of efficiency, R stores the content of a factor as a
vector of integers, which an integer is assigned to each of the possible
levels. Recall levels are assigned in alphabetical order. In this case,
the first item in our factor_snps object is “A”, which
happens to be the 1st level of our factor, ordered alphabetically. This
explains the sequence of “1”s (“Factor w/ 4 levels”A”,“C”,“G”,“T”: 1 1 1
1 1 1 1 1 1 1 …“), since”A” is the first level, and the first few items
in our factor are all “A”s.
We can see how many items in our vector fall into each category:
R
summary(factor_snps)
OUTPUT
A C G T
211 139 154 203 As you can imagine, this is already useful when you want to generate a tally.
Tip: treating objects as categories without changing their mode
You don’t have to make an object a factor to get the benefits of
treating an object as a factor. See what happens when you use the
as.factor() function on factor_snps. To
generate a tally, you can sometimes also use the table()
function; though sometimes you may need to combine both (i.e.
table(as.factor(object)))
Plotting and ordering factors
One of the most common uses for factors will be when you plot categorical values. For example, suppose we want to know how many of our variants had each possible SNP we could generate a plot:
R
plot(factor_snps)
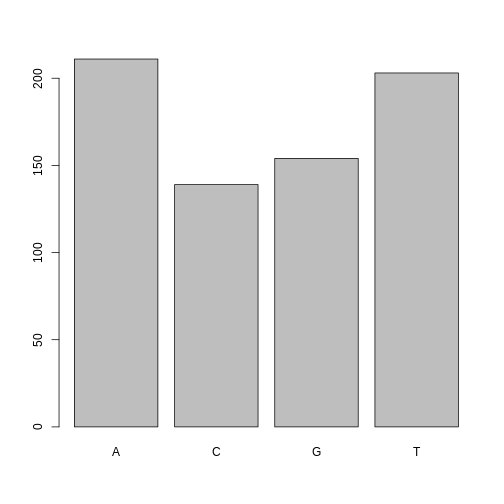
This isn’t a particularly pretty example of a plot but it works. We’ll be learning much more about creating nice, publication-quality graphics later in this lesson.
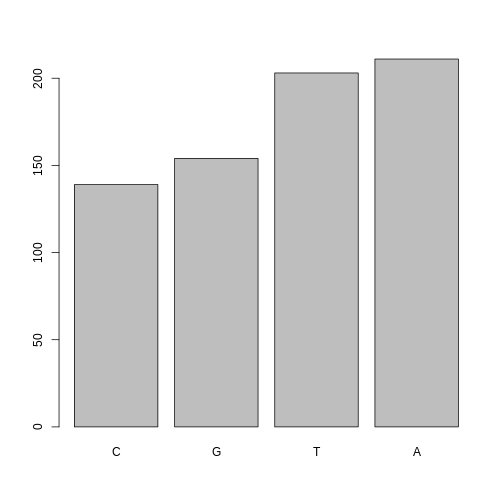
If you recall, factors are ordered alphabetically. That might make sense, but categories (e.g., “red”, “blue”, “green”) often do not have an intrinsic order. What if we wanted to order our plot according to the numerical value (i.e., in descending order of SNP frequency)? We can enforce an order on our factors:
R
ordered_factor_snps <- factor(factor_snps, levels = names(sort(table(factor_snps))))
Let’s deconstruct this from the inside out (you can try each of these commands to see why this works):
- We create a table of
factor_snpsto get the frequency of each SNP:table(factor_snps) - We sort this table:
sort(table(factor_snps)); use thedecreasing =parameter for this function if you wanted to change from the default of FALSE - Using the
namesfunction gives us just the character names of the table sorted by frequencies:names(sort(table(factor_snps))) - The
factorfunction is what allows us to create a factor. We give it thefactor_snpsobject as input, and use thelevels=parameter to enforce the ordering of the levels.
Now we see our plot has be reordered:
R
plot(ordered_factor_snps)
Factors come in handy in many places when using R. Even using more sophisticated plotting packages such as ggplot2 will sometimes require you to understand how to manipulate factors.
Tip: Packages in R – what are they and why do we use them?
Packages are simply collections of functions and/or data that can be
used to extend the capabilities of R beyond the core functionality that
comes with it by default. There are useful R packages available that
span all types of statistical analysis, data visualization, and more.
The main place that R packages are installed from is a website called CRAN (the Comprehensive R Archive
Network). Many thousands of R packages are available there, and when you
use the built-in R function install.packages(), it will
look for a CRAN repository to install from. So, for example, to install
tidyverse packages such as
dplyr and ggplot2 (which you’ll do in the next
few lessons), you would use the following command:
R
# install a package from CRAN
install.packages("ggplot2")
OUTPUT
The following package(s) will be installed:
- ggplot2 [3.4.2]
These packages will be installed into "~/work/genomics-r-intro/genomics-r-intro/renv/profiles/lesson-requirements/renv/library/R-4.3/x86_64-pc-linux-gnu".
# Installing packages --------------------------------------------------------
- Installing ggplot2 ... OK [linked from cache]
Successfully installed 1 package in 10 milliseconds.Subsetting data frames
Next, we are going to talk about how you can get specific values from data frames, and where necessary, change the mode of a column of values.
The first thing to remember is that a data frame is two-dimensional
(rows and columns). Therefore, to select a specific value we will will
once again use [] (bracket) notation, but we will specify
more than one value (except in some cases where we are taking a
range).
Exercise: Subsetting a data frame
Try the following indices and functions and try to figure out what they return
variants[1,1]variants[2,4]variants[801,29]variants[2, ]variants[-1, ]variants[1:4,1]variants[1:10,c("REF","ALT")]variants[,c("sample_id")]head(variants)tail(variants)variants$sample_idvariants[variants$REF == "A",]
R
variants[1,1]
OUTPUT
[1] "SRR2584863"R
variants[2,4]
OUTPUT
[1] NAR
variants[801,29]
OUTPUT
[1] "T"R
variants[2, ]
OUTPUT
sample_id CHROM POS ID REF ALT QUAL FILTER INDEL IDV IMF DP VDB
2 SRR2584863 CP000819.1 263235 NA G T 85 NA FALSE NA NA 6 0.096133
RPB MQB BQB MQSB SGB MQ0F ICB HOB AC AN DP4 MQ
2 1 1 1 NA -0.590765 0.166667 NA NA 1 1 0,1,0,5 33
Indiv gt_PL
2 /home/dcuser/dc_workshop/results/bam/SRR2584863.aligned.sorted.bam 112,0
gt_GT gt_GT_alleles
2 1 TR
variants[-1, ]
OUTPUT
sample_id CHROM POS ID REF ALT QUAL FILTER INDEL IDV IMF
2 SRR2584863 CP000819.1 263235 NA G T 85 NA FALSE NA NA
3 SRR2584863 CP000819.1 281923 NA G T 217 NA FALSE NA NA
4 SRR2584863 CP000819.1 433359 NA CTTTTTTT CTTTTTTTT 64 NA TRUE 12 1.0
5 SRR2584863 CP000819.1 473901 NA CCGC CCGCGC 228 NA TRUE 9 0.9
6 SRR2584863 CP000819.1 648692 NA C T 210 NA FALSE NA NA
7 SRR2584863 CP000819.1 1331794 NA C A 178 NA FALSE NA NA
DP VDB RPB MQB BQB MQSB SGB MQ0F ICB HOB AC AN DP4 MQ
2 6 0.096133 1 1 1 NA -0.590765 0.166667 NA NA 1 1 0,1,0,5 33
3 10 0.774083 NA NA NA 0.974597 -0.662043 0.000000 NA NA 1 1 0,0,4,5 60
4 12 0.477704 NA NA NA 1.000000 -0.676189 0.000000 NA NA 1 1 0,1,3,8 60
5 10 0.659505 NA NA NA 0.916482 -0.662043 0.000000 NA NA 1 1 1,0,2,7 60
6 10 0.268014 NA NA NA 0.916482 -0.670168 0.000000 NA NA 1 1 0,0,7,3 60
7 8 0.624078 NA NA NA 0.900802 -0.651104 0.000000 NA NA 1 1 0,0,3,5 60
Indiv gt_PL
2 /home/dcuser/dc_workshop/results/bam/SRR2584863.aligned.sorted.bam 112,0
3 /home/dcuser/dc_workshop/results/bam/SRR2584863.aligned.sorted.bam 247,0
4 /home/dcuser/dc_workshop/results/bam/SRR2584863.aligned.sorted.bam 91,0
5 /home/dcuser/dc_workshop/results/bam/SRR2584863.aligned.sorted.bam 255,0
6 /home/dcuser/dc_workshop/results/bam/SRR2584863.aligned.sorted.bam 240,0
7 /home/dcuser/dc_workshop/results/bam/SRR2584863.aligned.sorted.bam 208,0
gt_GT gt_GT_alleles
2 1 T
3 1 T
4 1 CTTTTTTTT
5 1 CCGCGC
6 1 T
7 1 AR
variants[1:4,1]
OUTPUT
[1] "SRR2584863" "SRR2584863" "SRR2584863" "SRR2584863"R
variants[1:10,c("REF","ALT")]
OUTPUT
REF
1 T
2 G
3 G
4 CTTTTTTT
5 CCGC
6 C
7 C
8 G
9 ACAGCCAGCCAGCCAGCCAGCCAGCCAGCCAG
10 AT
ALT
1 G
2 T
3 T
4 CTTTTTTTT
5 CCGCGC
6 T
7 A
8 A
9 ACAGCCAGCCAGCCAGCCAGCCAGCCAGCCAGCCAGCCAGCCAGCCAGCCAGCCAG
10 ATTR
variants[,c("sample_id")]
OUTPUT
[1] "SRR2584863" "SRR2584863" "SRR2584863" "SRR2584863" "SRR2584863"
[6] "SRR2584863"R
head(variants)
OUTPUT
sample_id CHROM POS ID REF ALT QUAL FILTER INDEL IDV IMF
1 SRR2584863 CP000819.1 9972 NA T G 91 NA FALSE NA NA
2 SRR2584863 CP000819.1 263235 NA G T 85 NA FALSE NA NA
3 SRR2584863 CP000819.1 281923 NA G T 217 NA FALSE NA NA
4 SRR2584863 CP000819.1 433359 NA CTTTTTTT CTTTTTTTT 64 NA TRUE 12 1.0
5 SRR2584863 CP000819.1 473901 NA CCGC CCGCGC 228 NA TRUE 9 0.9
6 SRR2584863 CP000819.1 648692 NA C T 210 NA FALSE NA NA
DP VDB RPB MQB BQB MQSB SGB MQ0F ICB HOB AC AN DP4 MQ
1 4 0.0257451 NA NA NA NA -0.556411 0.000000 NA NA 1 1 0,0,0,4 60
2 6 0.0961330 1 1 1 NA -0.590765 0.166667 NA NA 1 1 0,1,0,5 33
3 10 0.7740830 NA NA NA 0.974597 -0.662043 0.000000 NA NA 1 1 0,0,4,5 60
4 12 0.4777040 NA NA NA 1.000000 -0.676189 0.000000 NA NA 1 1 0,1,3,8 60
5 10 0.6595050 NA NA NA 0.916482 -0.662043 0.000000 NA NA 1 1 1,0,2,7 60
6 10 0.2680140 NA NA NA 0.916482 -0.670168 0.000000 NA NA 1 1 0,0,7,3 60
Indiv gt_PL
1 /home/dcuser/dc_workshop/results/bam/SRR2584863.aligned.sorted.bam 121,0
2 /home/dcuser/dc_workshop/results/bam/SRR2584863.aligned.sorted.bam 112,0
3 /home/dcuser/dc_workshop/results/bam/SRR2584863.aligned.sorted.bam 247,0
4 /home/dcuser/dc_workshop/results/bam/SRR2584863.aligned.sorted.bam 91,0
5 /home/dcuser/dc_workshop/results/bam/SRR2584863.aligned.sorted.bam 255,0
6 /home/dcuser/dc_workshop/results/bam/SRR2584863.aligned.sorted.bam 240,0
gt_GT gt_GT_alleles
1 1 G
2 1 T
3 1 T
4 1 CTTTTTTTT
5 1 CCGCGC
6 1 TR
tail(variants)
OUTPUT
sample_id CHROM POS ID REF ALT QUAL FILTER INDEL IDV IMF DP
796 SRR2589044 CP000819.1 3444175 NA G T 184 NA FALSE NA NA 9
797 SRR2589044 CP000819.1 3481820 NA A G 225 NA FALSE NA NA 12
798 SRR2589044 CP000819.1 3893550 NA AG AGG 101 NA TRUE 4 1 4
799 SRR2589044 CP000819.1 3901455 NA A AC 70 NA TRUE 3 1 3
800 SRR2589044 CP000819.1 4100183 NA A G 177 NA FALSE NA NA 8
801 SRR2589044 CP000819.1 4431393 NA TGG T 225 NA TRUE 10 1 10
VDB RPB MQB BQB MQSB SGB MQ0F ICB HOB AC AN DP4 MQ
796 0.4714620 NA NA NA 0.992367 -0.651104 0 NA NA 1 1 0,0,4,4 60
797 0.8707240 NA NA NA 1.000000 -0.680642 0 NA NA 1 1 0,0,4,8 60
798 0.9182970 NA NA NA 1.000000 -0.556411 0 NA NA 1 1 0,0,3,1 52
799 0.0221621 NA NA NA NA -0.511536 0 NA NA 1 1 0,0,3,0 60
800 0.9272700 NA NA NA 0.900802 -0.651104 0 NA NA 1 1 0,0,3,5 60
801 0.7488140 NA NA NA 1.007750 -0.670168 0 NA NA 1 1 0,0,4,6 60
Indiv gt_PL
796 /home/dcuser/dc_workshop/results/bam/SRR2589044.aligned.sorted.bam 214,0
797 /home/dcuser/dc_workshop/results/bam/SRR2589044.aligned.sorted.bam 255,0
798 /home/dcuser/dc_workshop/results/bam/SRR2589044.aligned.sorted.bam 131,0
799 /home/dcuser/dc_workshop/results/bam/SRR2589044.aligned.sorted.bam 100,0
800 /home/dcuser/dc_workshop/results/bam/SRR2589044.aligned.sorted.bam 207,0
801 /home/dcuser/dc_workshop/results/bam/SRR2589044.aligned.sorted.bam 255,0
gt_GT gt_GT_alleles
796 1 T
797 1 G
798 1 AGG
799 1 AC
800 1 G
801 1 TR
variants$sample_id
OUTPUT
[1] "SRR2584863" "SRR2584863" "SRR2584863" "SRR2584863" "SRR2584863"
[6] "SRR2584863"R
variants[variants$REF == "A",]
OUTPUT
sample_id CHROM POS ID REF ALT QUAL FILTER INDEL IDV IMF DP
11 SRR2584863 CP000819.1 2407766 NA A C 104 NA FALSE NA NA 9
12 SRR2584863 CP000819.1 2446984 NA A C 225 NA FALSE NA NA 20
14 SRR2584863 CP000819.1 2665639 NA A T 225 NA FALSE NA NA 19
16 SRR2584863 CP000819.1 3339313 NA A C 211 NA FALSE NA NA 10
18 SRR2584863 CP000819.1 3481820 NA A G 200 NA FALSE NA NA 9
19 SRR2584863 CP000819.1 3488669 NA A C 225 NA FALSE NA NA 13
VDB RPB MQB BQB MQSB SGB MQ0F ICB HOB AC
11 0.0230738 0.900802 0.150134 0.750668 0.500000 -0.590765 0.333333 NA NA 1
12 0.0714027 NA NA NA 1.000000 -0.689466 0.000000 NA NA 1
14 0.9960390 NA NA NA 1.000000 -0.690438 0.000000 NA NA 1
16 0.4059360 NA NA NA 1.007750 -0.670168 0.000000 NA NA 1
18 0.1070810 NA NA NA 0.974597 -0.662043 0.000000 NA NA 1
19 0.0162706 NA NA NA 1.000000 -0.680642 0.000000 NA NA 1
AN DP4 MQ
11 1 3,0,3,2 25
12 1 0,0,10,6 60
14 1 0,0,12,5 60
16 1 0,0,4,6 60
18 1 0,0,4,5 60
19 1 0,0,8,4 60
Indiv gt_PL
11 /home/dcuser/dc_workshop/results/bam/SRR2584863.aligned.sorted.bam 131,0
12 /home/dcuser/dc_workshop/results/bam/SRR2584863.aligned.sorted.bam 255,0
14 /home/dcuser/dc_workshop/results/bam/SRR2584863.aligned.sorted.bam 255,0
16 /home/dcuser/dc_workshop/results/bam/SRR2584863.aligned.sorted.bam 241,0
18 /home/dcuser/dc_workshop/results/bam/SRR2584863.aligned.sorted.bam 230,0
19 /home/dcuser/dc_workshop/results/bam/SRR2584863.aligned.sorted.bam 255,0
gt_GT gt_GT_alleles
11 1 C
12 1 C
14 1 T
16 1 C
18 1 G
19 1 CThe subsetting notation is very similar to what we learned for vectors. The key differences include:
- Typically provide two values separated by commas: data.frame[row, column]
- In cases where you are taking a continuous range of numbers use a colon between the numbers (start:stop, inclusive)
- For a non continuous set of numbers, pass a vector using
c() - Index using the name of a column(s) by passing them as vectors using
c()
Finally, in all of the subsetting exercises above, we printed values to the screen. You can create a new data frame object by assigning them to a new object name:
R
# create a new data frame containing only observations from SRR2584863
SRR2584863_variants <- variants[variants$sample_id == "SRR2584863",]
# check the dimension of the data frame
dim(SRR2584863_variants)
OUTPUT
[1] 25 29R
# get a summary of the data frame
summary(SRR2584863_variants)
OUTPUT
sample_id CHROM POS ID
Length:25 Length:25 Min. : 9972 Mode:logical
Class :character Class :character 1st Qu.:1331794 NA's:25
Mode :character Mode :character Median :2618472
Mean :2464989
3rd Qu.:3488669
Max. :4616538
REF ALT QUAL FILTER
Length:25 Length:25 Min. : 31.89 Mode:logical
Class :character Class :character 1st Qu.:104.00 NA's:25
Mode :character Mode :character Median :211.00
Mean :172.97
3rd Qu.:225.00
Max. :228.00
INDEL IDV IMF DP
Mode :logical Min. : 2.00 Min. :0.6667 Min. : 2.0
FALSE:19 1st Qu.: 3.25 1st Qu.:0.9250 1st Qu.: 9.0
TRUE :6 Median : 8.00 Median :1.0000 Median :10.0
Mean : 7.00 Mean :0.9278 Mean :10.4
3rd Qu.: 9.75 3rd Qu.:1.0000 3rd Qu.:12.0
Max. :12.00 Max. :1.0000 Max. :20.0
NA's :19 NA's :19
VDB RPB MQB BQB
Min. :0.01627 Min. :0.9008 Min. :0.04979 Min. :0.7507
1st Qu.:0.07140 1st Qu.:0.9275 1st Qu.:0.09996 1st Qu.:0.7627
Median :0.37674 Median :0.9542 Median :0.15013 Median :0.7748
Mean :0.40429 Mean :0.9517 Mean :0.39997 Mean :0.8418
3rd Qu.:0.65951 3rd Qu.:0.9771 3rd Qu.:0.57507 3rd Qu.:0.8874
Max. :0.99604 Max. :1.0000 Max. :1.00000 Max. :1.0000
NA's :22 NA's :22 NA's :22
MQSB SGB MQ0F ICB
Min. :0.5000 Min. :-0.6904 Min. :0.00000 Mode:logical
1st Qu.:0.9599 1st Qu.:-0.6762 1st Qu.:0.00000 NA's:25
Median :0.9962 Median :-0.6620 Median :0.00000
Mean :0.9442 Mean :-0.6341 Mean :0.04667
3rd Qu.:1.0000 3rd Qu.:-0.6168 3rd Qu.:0.00000
Max. :1.0128 Max. :-0.4536 Max. :0.66667
NA's :3
HOB AC AN DP4 MQ
Mode:logical Min. :1 Min. :1 Length:25 Min. :10.00
NA's:25 1st Qu.:1 1st Qu.:1 Class :character 1st Qu.:60.00
Median :1 Median :1 Mode :character Median :60.00
Mean :1 Mean :1 Mean :55.52
3rd Qu.:1 3rd Qu.:1 3rd Qu.:60.00
Max. :1 Max. :1 Max. :60.00
Indiv gt_PL gt_GT gt_GT_alleles
Length:25 Length:25 Min. :1 Length:25
Class :character Class :character 1st Qu.:1 Class :character
Mode :character Mode :character Median :1 Mode :character
Mean :1
3rd Qu.:1
Max. :1
Coercing values in data frames
Sometimes, it is possible that R will misinterpret the type of data represented in a data frame, or store that data in a mode which prevents you from operating on the data the way you wish. For example, a long list of gene names isn’t usually thought of as a categorical variable, the way that your experimental condition (e.g. control, treatment) might be. More importantly, some R packages you use to analyze your data may expect characters as input, not factors. At other times (such as plotting or some statistical analyses) a factor may be more appropriate. Ultimately, you should know how to change the mode of an object.
First, its very important to recognize that coercion happens in R all the time. This can be a good thing when R gets it right, or a bad thing when the result is not what you expect. Consider:
R
snp_chromosomes <- c('3', '11', 'X', '6')
typeof(snp_chromosomes)
OUTPUT
[1] "character"Although there are several numbers in our vector, they are all in quotes, so we have explicitly told R to consider them as characters. However, even if we removed the quotes from the numbers, R would coerce everything into a character:
R
snp_chromosomes_2 <- c(3, 11, 'X', 6)
typeof(snp_chromosomes_2)
OUTPUT
[1] "character"R
snp_chromosomes_2[1]
OUTPUT
[1] "3"We can use the as. functions to explicitly coerce values
from one form into another. Consider the following vector of characters,
which all happen to be valid numbers:
R
snp_positions_2 <- c("8762685", "66560624", "67545785", "154039662")
typeof(snp_positions_2)
OUTPUT
[1] "character"R
snp_positions_2[1]
OUTPUT
[1] "8762685"Now we can coerce snp_positions_2 into a numeric type
using as.numeric():
R
snp_positions_2 <- as.numeric(snp_positions_2)
typeof(snp_positions_2)
OUTPUT
[1] "double"R
snp_positions_2[1]
OUTPUT
[1] 8762685Sometimes coercion is straight forward, but what would happen if we
tried using as.numeric() on
snp_chromosomes_2
R
snp_chromosomes_2 <- as.numeric(snp_chromosomes_2)
WARNING
Warning: NAs introduced by coercionIf we check, we will see that an NA value (R’s default
value for missing data) has been introduced.
R
snp_chromosomes_2
OUTPUT
[1] 3 11 NA 6Trouble can really start when we try to coerce a factor. For example,
when we try to coerce the sample_id column in our data
frame into a numeric mode look at the result:
R
as.numeric(variants$sample_id)
WARNING
Warning: NAs introduced by coercionOUTPUT
[1] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[26] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[51] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[76] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[101] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[126] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[151] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[176] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[201] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[226] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[251] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[276] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[301] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[326] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[351] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[376] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[401] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[426] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[451] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[476] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[501] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[526] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[551] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[576] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[601] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[626] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[651] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[676] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[701] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[726] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[751] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[776] NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA
[801] NAStrangely, it works! Almost. Instead of giving an error message, R returns numeric values, which in this case are the integers assigned to the levels in this factor. This kind of behavior can lead to hard-to-find bugs, for example when we do have numbers in a factor, and we get numbers from a coercion. If we don’t look carefully, we may not notice a problem.
If you need to coerce an entire column you can overwrite it using an expression like this one:
R
# make the 'REF' column a character type column
variants$REF <- as.character(variants$REF)
# check the type of the column
typeof(variants$REF)
OUTPUT
[1] "character"StringsAsFactors = ?
Lets summarize this section on coercion with a few take home messages.
- When you explicitly coerce one data type into another (this is known as explicit coercion), be careful to check the result. Ideally, you should try to see if its possible to avoid steps in your analysis that force you to coerce.
- R will sometimes coerce without you asking for it. This is called (appropriately) implicit coercion. For example when we tried to create a vector with multiple data types, R chose one type through implicit coercion.
- Check the structure (
str()) of your data frames before working with them!
Tip: coercion isn’t limited to data frames
Prior to R 4.0 when importing a data frame using any one of the
read.table() functions such as read.csv() ,
the argument StringsAsFactors was by default set to true
TRUE. Setting it to FALSE will treat any non-numeric column to a
character type. read.csv() documentation, you will also see
you can explicitly type your columns using the colClasses
argument. Other R packages (such as the Tidyverse “readr”) don’t have
this particular conversion issue, but many packages will still try to
guess a data type.
Data frame bonus material: math, sorting, renaming
Here are a few operations that don’t need much explanation, but which are good to know.
There are lots of arithmetic functions you may want to apply to your data frame, covering those would be a course in itself (there is some starting material here). Our lessons will cover some additional summary statistical functions in a subsequent lesson, but overall we will focus on data cleaning and visualization.
You can use functions like mean(), min(),
max() on an individual column. Let’s look at the “DP” or
filtered depth. This value shows the number of filtered reads that
support each of the reported variants.
R
max(variants$DP)
OUTPUT
[1] 79You can sort a data frame using the order()
function:
R
sorted_by_DP <- variants[order(variants$DP), ]
head(sorted_by_DP$DP)
OUTPUT
[1] 2 2 2 2 2 2R
sorted_by_DP <- variants[order(variants$DP, decreasing = TRUE), ]
head(sorted_by_DP$DP)
OUTPUT
[1] 79 46 41 29 29 27You can rename columns:
R
colnames(variants)[colnames(variants) == "sample_id"] <- "strain"
# check the column name (hint names are returned as a vector)
colnames(variants)
OUTPUT
[1] "strain" "CHROM" "POS" "ID"
[5] "REF" "ALT" "QUAL" "FILTER"
[9] "INDEL" "IDV" "IMF" "DP"
[13] "VDB" "RPB" "MQB" "BQB"
[17] "MQSB" "SGB" "MQ0F" "ICB"
[21] "HOB" "AC" "AN" "DP4"
[25] "MQ" "Indiv" "gt_PL" "gt_GT"
[29] "gt_GT_alleles"Saving your data frame to a file
We can save data to a file. We will save our
SRR2584863_variants object to a .csv file using the
write.csv() function:
R
write.csv(SRR2584863_variants, file = "data/SRR2584863_variants.csv")
The write.csv() function has some additional arguments
listed in the help, but at a minimum you need to tell it what data frame
to write to file, and give a path to a file name in quotes (if you only
provide a file name, the file will be written in the current working
directory).
Importing data from Excel
Excel is one of the most common formats, so we need to discuss how to make these files play nicely with R. The simplest way to import data from Excel is to save your Excel file in .csv format*. You can then import into R right away. Sometimes you may not be able to do this (imagine you have data in 300 Excel files, are you going to open and export all of them?).
One common R package (a set of code with features you can download and add to your R installation) is the readxl package which can open and import Excel files. Rather than addressing package installation this second (we’ll discuss this soon!), we can take advantage of RStudio’s import feature which integrates this package. (Note: this feature is available only in the latest versions of RStudio such as is installed on our cloud instance).
First, in the RStudio menu go to File, select Import Dataset, and choose From Excel… (notice there are several other options you can explore).

Next, under File/Url: click the Browse
button and navigate to the Ecoli_metadata.xlsx file
located at /home/dcuser/dc_sample_data/R. You should now
see a preview of the data to be imported:

Notice that you have the option to change the data type of each variable by clicking arrow (drop-down menu) next to each column title. Under Import Options you may also rename the data, choose a different sheet to import, and choose how you will handle headers and skipped rows. Under Code Preview you can see the code that will be used to import this file. We could have written this code and imported the Excel file without the RStudio import function, but now you can choose your preference.
In this exercise, we will leave the title of the data frame as Ecoli_metadata, and there are no other options we need to adjust. Click the Import button to import the data.
Finally, let’s check the first few lines of the
Ecoli_metadata data frame:
R
head(Ecoli_metadata)
OUTPUT
# A tibble: 6 × 7
sample generation clade strain cit run genome_size
<chr> <dbl> <chr> <chr> <chr> <chr> <dbl>
1 REL606 0 NA REL606 unknown <NA> 4.62
2 REL1166A 2000 unknown REL606 unknown SRR098028 4.63
3 ZDB409 5000 unknown REL606 unknown SRR098281 4.6
4 ZDB429 10000 UC REL606 unknown SRR098282 4.59
5 ZDB446 15000 UC REL606 unknown SRR098283 4.66
6 ZDB458 20000 (C1,C2) REL606 unknown SRR098284 4.63The type of this object is ‘tibble’, a type of data frame we will
talk more about in the ‘dplyr’ section. If you needed a true R data
frame you could coerce with as.data.frame().
Exercise: Putting it all together - data frames
Using the Ecoli_metadata data frame created
above, answer the following questions
What are the dimensions (# rows, # columns) of the data frame?
What are categories are there in the
citcolumn? hint: treat column as factorHow many of each of the
citcategories are there?What is the genome size for the 7th observation in this data set?
What is the median value of the variable
genome_sizeRename the column
sampletosample_idCreate a new column (name genome_size_bp) and set it equal to the genome_size multiplied by 1,000,000
Save the edited Ecoli_metadata data frame as “exercise_solution.csv” in your current working directory.
R
dim(Ecoli_metadata)
OUTPUT
[1] 30 7R
levels(as.factor(Ecoli_metadata$cit))
OUTPUT
[1] "minus" "plus" "unknown"R
table(as.factor(Ecoli_metadata$cit))
OUTPUT
minus plus unknown
9 9 12 R
Ecoli_metadata[7,7]
OUTPUT
# A tibble: 1 × 1
genome_size
<dbl>
1 4.62R
median(Ecoli_metadata$genome_size)
OUTPUT
[1] 4.625R
colnames(Ecoli_metadata)[colnames(Ecoli_metadata) == "sample"] <- "sample_id"
Ecoli_metadata$genome_size_bp <- Ecoli_metadata$genome_size * 1000000
write.csv(Ecoli_metadata, file = "exercise_solution.csv")
Keypoints
- It is easy to import data into R from tabular formats including Excel. However, you still need to check that R has imported and interpreted your data correctly
- There are best practices for organizing your data (keeping it tidy) and R is great for this
- Base R has many useful functions for manipulating your data, but all of R’s capabilities are greatly enhanced by software packages developed by the community
Content from Using packages from Bioconductor
Last updated on 2023-08-15 | Edit this page
Estimated time 13 minutes
Overview
Questions
- How do I use packages from the Bioconductor repository?
Objectives
- Describe what the Bioconductor repository is and what it is used for
- Describe how Bioconductor differs from CRAN
- Search Bioconductor for relevant packages
- Install a package from Bioconductor
Installing packages from somewhere else besides CRAN?
So far we have told you about using packages that are included in the base installation of R (this is what comes with R ‘out of the box’), and packages that you can install from CRAN (the Comprehensive R Archive Network), which is the primary place many people look for supplemental R packages to install. However, not all R packages are available on CRAN. For bioinformatics-related packages in particular, there is another repository that has many powerful packages that you can install. It is called Bioconductor and it is a repository specifically focused on bioinformatics packages. Bioconductor has a mission of “promot[ing] the statistical analysis and comprehension of current and emerging high-throughput biological assays.” This means that many if not all of the packages available on Bioconductor are focused on the analysis of biological data, and that it can be a great place to look for tools to help you analyze your -omics datasets!
So how do I use it?
Since access to the Bioconductor repository is not built in to base R ‘out of the box’, there are a couple steps needed to install packages from this alternative source. We will work through the steps (only 2!) to install a package to help with the VCF analysis we are working on, but you can use the same approach to install any of the many thousands of available packages.
First, install the BiocManager package
The first step is to install a package that is on CRAN,
BiocManager. This package will allow us to use it to
install packages from Bioconductor. You can think of Bioconductor kind
of like an alternative app store for your phone, except instead of apps
you are installing packages, and instead of your phone it’s your local R
package library.
R
# install the BiocManager from CRAN using the base R install.packages() function
install.packages("BiocManager")
To check if this worked (and also so you can make a note of the
version for reproducibility purposes), you can run
BiocManager::version() and it should give you the version
number.
R
# to make sure it worked, check the version
BiocManager::version()
Second, install the vcfR package from Bioconductor using
BiocManager
R
# install the vcfR package from bioconductor using BiocManager::install()
BiocManager::install("vcfR")
Depending on your particular system, you may need to also allow it to install some dependencies or update installed packages in order to successfully complete the process.
Note: Installing packages from Bioconductor vs from CRAN
Some packages begin by being available only on Bioconductor, and then
later move to CRAN. vcfR is one such package, which
originally was only available from Bioconductor, but is currently
available from CRAN. The other thing to know is that
BiocManager::install() will also install packages from CRAN
(it is a wrapper around install.packages() that adds some
extra features). There are other benefits to using
BiocManager::install() for Bioconductor packages, many of
which are outlined here. In short,
Bioconductor packages have a release cycle that is different from CRAN
and the install() function is aware of that difference, so
it helps to keep package versions in line with one another in a way that
doesn’t generally happen with the base R
install.packages().
Search for Bioconductor packages based on your analysis needs
While we are only focusing in this workshop on VCF analyses, there are hundreds or thousands of different types of data and analyses that bioinformaticians may want to work with. Sometimes you may get a new dataset and not know exactly where to start with analyzing or visualizing it. The Bioconductor package search view can be a great way to browse through the packages that are available.
Tip: Searching for packages on the Bioconductor website
There are several thousand packages available through the Bioconductor website. It can be a bit of a challenge to find what you want, but one helpful resource is the package search page.
In bioinformatics, there are often many different tools that can be
used in a particular instance. The authors of vcfR have compiled
some of them. One of those packages that is
available from Bioconductor is called VariantAnnotation
and may also be of interest to those working with vcf files in R.
Challenge
- Use the
BiocManager::available()function to see what packages are available matching a search term. - Use the biocViews interface to search for packages of interest.
You may or may not want to try installing the package, since not all dependencies always install easily. However, this will at least let you see what is available.
Resources
Keypoints
- Bioconductor is an alternative package repository for bioinformatics packages.
- Installing packages from Bioconductor requires a new method, since
it is not compatible with the
install.packages()function used for CRAN. - Check Bioconductor to see if there is a package relevant to your analysis before writing code yourself.
Content from Data Wrangling and Analyses with Tidyverse
Last updated on 2023-08-15 | Edit this page
Estimated time 55 minutes
Overview
Questions
- How can I manipulate data frames without repeating myself?
Objectives
- Describe what the
dplyrpackage in R is used for. - Apply common
dplyrfunctions to manipulate data in R. - Employ the ‘pipe’ operator to link together a sequence of functions.
- Employ the ‘mutate’ function to apply other chosen functions to existing columns and create new columns of data.
- Employ the ‘split-apply-combine’ concept to split the data into groups, apply analysis to each group, and combine the results.
Bracket subsetting is handy, but it can be cumbersome and difficult to read, especially for complicated operations.
Luckily, the dplyr
package provides a number of very useful functions for manipulating data
frames in a way that will reduce repetition, reduce the probability of
making errors, and probably even save you some typing. As an added
bonus, you might even find the dplyr grammar easier to
read.
Here we’re going to cover some of the most commonly used functions as
well as using pipes (%>%) to combine them:
glimpse()select()filter()group_by()summarize()mutate()-
pivot_longerandpivot_wider
Packages in R are sets of additional functions that let you do more
stuff in R. The functions we’ve been using, like str(),
come built into R; packages give you access to more functions. You need
to install a package and then load it to be able to use it.
R
install.packages("dplyr") ## installs dplyr package
install.packages("tidyr") ## installs tidyr package
install.packages("ggplot2") ## installs ggplot2 package
install.packages("readr") ## install readr package
You might get asked to choose a CRAN mirror – this is asking you to choose a site to download the package from. The choice doesn’t matter too much; I’d recommend choosing the RStudio mirror.
R
library("dplyr") ## loads in dplyr package to use
library("tidyr") ## loads in tidyr package to use
library("ggplot2") ## loads in ggplot2 package to use
library("readr") ## load in readr package to use
You only need to install a package once per computer, but you need to load it every time you open a new R session and want to use that package.
Tip: Installing packages
It may be temping to install the tidyverse package, as
it contains many useful collection of packages for this lesson and
beyond. However, when teaching or following this lesson, we advise that
participants install dplyr, readr,
ggplot2, and tidyr individually as shown
above. Otherwise, a substaial amount of the lesson will be spend waiting
for the installation to complete.
What is dplyr?
The package dplyr is a fairly new (2014) package that
tries to provide easy tools for the most common data manipulation tasks.
This package is also included in the tidyverse package,
which is a collection of eight different packages (dplyr,
ggplot2, tibble, tidyr,
readr, purrr, stringr, and
forcats). It is built to work directly with data frames.
The thinking behind it was largely inspired by the package
plyr which has been in use for some time but suffered from
being slow in some cases.dplyr addresses this by porting
much of the computation to C++. An additional feature is the ability to
work with data stored directly in an external database. The benefits of
doing this are that the data can be managed natively in a relational
database, queries can be conducted on that database, and only the
results of the query returned.
This addresses a common problem with R in that all operations are conducted in memory and thus the amount of data you can work with is limited by available memory. The database connections essentially remove that limitation in that you can have a database that is over 100s of GB, conduct queries on it directly and pull back just what you need for analysis in R.
Loading .csv files in tidy style
The Tidyverse’s readr package provides its own unique
way of loading .csv files in to R using read_csv(), which
is similar to read.csv(). read_csv() allows
users to load in their data faster, doesn’t create row names, and allows
you to access non-standard variable names (ie. variables that start with
numbers of contain spaces), and outputs your data on the R console in a
tidier way. In short, it’s a much friendlier way of loading in
potentially messy data.
Now let’s load our vcf .csv file using read_csv():
Taking a quick look at data frames
Similar to str(), which comes built into R,
glimpse() is a dplyr function that (as the
name suggests) gives a glimpse of the data frame.
OUTPUT
Rows: 801
Columns: 29
$ sample_id <chr> "SRR2584863", "SRR2584863", "SRR2584863", "SRR2584863", …
$ CHROM <chr> "CP000819.1", "CP000819.1", "CP000819.1", "CP000819.1", …
$ POS <dbl> 9972, 263235, 281923, 433359, 473901, 648692, 1331794, 1…
$ ID <lgl> NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, …
$ REF <chr> "T", "G", "G", "CTTTTTTT", "CCGC", "C", "C", "G", "ACAGC…
$ ALT <chr> "G", "T", "T", "CTTTTTTTT", "CCGCGC", "T", "A", "A", "AC…
$ QUAL <dbl> 91.0000, 85.0000, 217.0000, 64.0000, 228.0000, 210.0000,…
$ FILTER <lgl> NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, …
$ INDEL <lgl> FALSE, FALSE, FALSE, TRUE, TRUE, FALSE, FALSE, FALSE, TR…
$ IDV <dbl> NA, NA, NA, 12, 9, NA, NA, NA, 2, 7, NA, NA, NA, NA, NA,…
$ IMF <dbl> NA, NA, NA, 1.000000, 0.900000, NA, NA, NA, 0.666667, 1.…
$ DP <dbl> 4, 6, 10, 12, 10, 10, 8, 11, 3, 7, 9, 20, 12, 19, 15, 10…
$ VDB <dbl> 0.0257451, 0.0961330, 0.7740830, 0.4777040, 0.6595050, 0…
$ RPB <dbl> NA, 1.000000, NA, NA, NA, NA, NA, NA, NA, NA, 0.900802, …
$ MQB <dbl> NA, 1.0000000, NA, NA, NA, NA, NA, NA, NA, NA, 0.1501340…
$ BQB <dbl> NA, 1.000000, NA, NA, NA, NA, NA, NA, NA, NA, 0.750668, …
$ MQSB <dbl> NA, NA, 0.974597, 1.000000, 0.916482, 0.916482, 0.900802…
$ SGB <dbl> -0.556411, -0.590765, -0.662043, -0.676189, -0.662043, -…
$ MQ0F <dbl> 0.000000, 0.166667, 0.000000, 0.000000, 0.000000, 0.0000…
$ ICB <lgl> NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, …
$ HOB <lgl> NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, …
$ AC <dbl> 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1,…
$ AN <dbl> 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1,…
$ DP4 <chr> "0,0,0,4", "0,1,0,5", "0,0,4,5", "0,1,3,8", "1,0,2,7", "…
$ MQ <dbl> 60, 33, 60, 60, 60, 60, 60, 60, 60, 60, 25, 60, 10, 60, …
$ Indiv <chr> "/home/dcuser/dc_workshop/results/bam/SRR2584863.aligned…
$ gt_PL <dbl> 1210, 1120, 2470, 910, 2550, 2400, 2080, 2550, 11128, 19…
$ gt_GT <dbl> 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1,…
$ gt_GT_alleles <chr> "G", "T", "T", "CTTTTTTTT", "CCGCGC", "T", "A", "A", "AC…In the above output, we can already gather some information about
variants, such as the number of rows and columns, column
names, type of vector in the columns, and the first few entries of each
column. Although what we see is similar to outputs of
str(), this method gives a cleaner visual output.
Selecting columns and filtering rows
To select columns of a data frame, use select(). The
first argument to this function is the data frame
(variants), and the subsequent arguments are the columns to
keep.
R
select(variants, sample_id, REF, ALT, DP)
OUTPUT
# A tibble: 801 × 4
sample_id REF ALT DP
<chr> <chr> <chr> <dbl>
1 SRR2584863 T G 4
2 SRR2584863 G T 6
3 SRR2584863 G T 10
4 SRR2584863 CTTTTTTT CTTTTTTTT 12
5 SRR2584863 CCGC CCGCGC 10
6 SRR2584863 C T 10
7 SRR2584863 C A 8
8 SRR2584863 G A 11
9 SRR2584863 ACAGCCAGCCAGCCAGCCAGCCAGCCAGCCAG ACAGCCAGCCAGCCAGCCAGCCAGCC… 3
10 SRR2584863 AT ATT 7
# ℹ 791 more rowsTo select all columns except certain ones, put a “-” in front of the variable to exclude it.
R
select(variants, -CHROM)
OUTPUT
# A tibble: 801 × 28
sample_id POS ID REF ALT QUAL FILTER INDEL IDV IMF DP
<chr> <dbl> <lgl> <chr> <chr> <dbl> <lgl> <lgl> <dbl> <dbl> <dbl>
1 SRR2584863 9972 NA T G 91 NA FALSE NA NA 4
2 SRR2584863 263235 NA G T 85 NA FALSE NA NA 6
3 SRR2584863 281923 NA G T 217 NA FALSE NA NA 10
4 SRR2584863 433359 NA CTTTTTTT CTTT… 64 NA TRUE 12 1 12
5 SRR2584863 473901 NA CCGC CCGC… 228 NA TRUE 9 0.9 10
6 SRR2584863 648692 NA C T 210 NA FALSE NA NA 10
7 SRR2584863 1331794 NA C A 178 NA FALSE NA NA 8
8 SRR2584863 1733343 NA G A 225 NA FALSE NA NA 11
9 SRR2584863 2103887 NA ACAGCCA… ACAG… 56 NA TRUE 2 0.667 3
10 SRR2584863 2333538 NA AT ATT 167 NA TRUE 7 1 7
# ℹ 791 more rows
# ℹ 17 more variables: VDB <dbl>, RPB <dbl>, MQB <dbl>, BQB <dbl>, MQSB <dbl>,
# SGB <dbl>, MQ0F <dbl>, ICB <lgl>, HOB <lgl>, AC <dbl>, AN <dbl>, DP4 <chr>,
# MQ <dbl>, Indiv <chr>, gt_PL <dbl>, gt_GT <dbl>, gt_GT_alleles <chr>dplyr also provides useful functions to select columns
based on their names. For instance, ends_with() allows you
to select columns that ends with specific letters. For instance, if you
wanted to select columns that end with the letter “B”:
R
select(variants, ends_with("B"))
OUTPUT
# A tibble: 801 × 8
VDB RPB MQB BQB MQSB SGB ICB HOB
<dbl> <dbl> <dbl> <dbl> <dbl> <dbl> <lgl> <lgl>
1 0.0257 NA NA NA NA -0.556 NA NA
2 0.0961 1 1 1 NA -0.591 NA NA
3 0.774 NA NA NA 0.975 -0.662 NA NA
4 0.478 NA NA NA 1 -0.676 NA NA
5 0.660 NA NA NA 0.916 -0.662 NA NA
6 0.268 NA NA NA 0.916 -0.670 NA NA
7 0.624 NA NA NA 0.901 -0.651 NA NA
8 0.992 NA NA NA 1.01 -0.670 NA NA
9 0.902 NA NA NA 1 -0.454 NA NA
10 0.568 NA NA NA 1.01 -0.617 NA NA
# ℹ 791 more rowsChallenge
Create a table that contains all the columns with the letter “i” and
column “POS”, without columns “Indiv” and “FILTER”. Hint: look at for a
function called contains(), which can be found in the help
documentation for ends with we just covered (?ends_with).
Note that contains() is not case sensistive.
R
# First, we select "POS" and all columns with letter "i". This will contain columns Indiv and FILTER.
variants_subset <- select(variants, POS, contains("i"))
# Next, we remove columns Indiv and FILTER
variants_result <- select(variants_subset, -Indiv, -FILTER)
variants_result
OUTPUT
# A tibble: 801 × 7
POS sample_id ID INDEL IDV IMF ICB
<dbl> <chr> <lgl> <lgl> <dbl> <dbl> <lgl>
1 9972 SRR2584863 NA FALSE NA NA NA
2 263235 SRR2584863 NA FALSE NA NA NA
3 281923 SRR2584863 NA FALSE NA NA NA
4 433359 SRR2584863 NA TRUE 12 1 NA
5 473901 SRR2584863 NA TRUE 9 0.9 NA
6 648692 SRR2584863 NA FALSE NA NA NA
7 1331794 SRR2584863 NA FALSE NA NA NA
8 1733343 SRR2584863 NA FALSE NA NA NA
9 2103887 SRR2584863 NA TRUE 2 0.667 NA
10 2333538 SRR2584863 NA TRUE 7 1 NA
# ℹ 791 more rowsR
variants_result <- select(variants, POS, contains("i"), -Indiv, -FILTER)
variants_result
OUTPUT
# A tibble: 801 × 7
POS sample_id ID INDEL IDV IMF ICB
<dbl> <chr> <lgl> <lgl> <dbl> <dbl> <lgl>
1 9972 SRR2584863 NA FALSE NA NA NA
2 263235 SRR2584863 NA FALSE NA NA NA
3 281923 SRR2584863 NA FALSE NA NA NA
4 433359 SRR2584863 NA TRUE 12 1 NA
5 473901 SRR2584863 NA TRUE 9 0.9 NA
6 648692 SRR2584863 NA FALSE NA NA NA
7 1331794 SRR2584863 NA FALSE NA NA NA
8 1733343 SRR2584863 NA FALSE NA NA NA
9 2103887 SRR2584863 NA TRUE 2 0.667 NA
10 2333538 SRR2584863 NA TRUE 7 1 NA
# ℹ 791 more rowsTo choose rows, use filter():
R
filter(variants, sample_id == "SRR2584863")
OUTPUT
# A tibble: 25 × 29
sample_id CHROM POS ID REF ALT QUAL FILTER INDEL IDV IMF
<chr> <chr> <dbl> <lgl> <chr> <chr> <dbl> <lgl> <lgl> <dbl> <dbl>
1 SRR2584863 CP000819… 9.97e3 NA T G 91 NA FALSE NA NA
2 SRR2584863 CP000819… 2.63e5 NA G T 85 NA FALSE NA NA
3 SRR2584863 CP000819… 2.82e5 NA G T 217 NA FALSE NA NA
4 SRR2584863 CP000819… 4.33e5 NA CTTT… CTTT… 64 NA TRUE 12 1
5 SRR2584863 CP000819… 4.74e5 NA CCGC CCGC… 228 NA TRUE 9 0.9
6 SRR2584863 CP000819… 6.49e5 NA C T 210 NA FALSE NA NA
7 SRR2584863 CP000819… 1.33e6 NA C A 178 NA FALSE NA NA
8 SRR2584863 CP000819… 1.73e6 NA G A 225 NA FALSE NA NA
9 SRR2584863 CP000819… 2.10e6 NA ACAG… ACAG… 56 NA TRUE 2 0.667
10 SRR2584863 CP000819… 2.33e6 NA AT ATT 167 NA TRUE 7 1
# ℹ 15 more rows
# ℹ 18 more variables: DP <dbl>, VDB <dbl>, RPB <dbl>, MQB <dbl>, BQB <dbl>,
# MQSB <dbl>, SGB <dbl>, MQ0F <dbl>, ICB <lgl>, HOB <lgl>, AC <dbl>,
# AN <dbl>, DP4 <chr>, MQ <dbl>, Indiv <chr>, gt_PL <dbl>, gt_GT <dbl>,
# gt_GT_alleles <chr>filter() will keep all the rows that match the
conditions that are provided. Here are a few examples:
R
# rows for which the reference genome has T or G
filter(variants, REF %in% c("T", "G"))
OUTPUT
# A tibble: 340 × 29
sample_id CHROM POS ID REF ALT QUAL FILTER INDEL IDV IMF DP
<chr> <chr> <dbl> <lgl> <chr> <chr> <dbl> <lgl> <lgl> <dbl> <dbl> <dbl>
1 SRR25848… CP00… 9.97e3 NA T G 91 NA FALSE NA NA 4
2 SRR25848… CP00… 2.63e5 NA G T 85 NA FALSE NA NA 6
3 SRR25848… CP00… 2.82e5 NA G T 217 NA FALSE NA NA 10
4 SRR25848… CP00… 1.73e6 NA G A 225 NA FALSE NA NA 11
5 SRR25848… CP00… 2.62e6 NA G T 31.9 NA FALSE NA NA 12
6 SRR25848… CP00… 3.00e6 NA G A 225 NA FALSE NA NA 15
7 SRR25848… CP00… 3.91e6 NA G T 225 NA FALSE NA NA 10
8 SRR25848… CP00… 9.97e3 NA T G 214 NA FALSE NA NA 10
9 SRR25848… CP00… 1.06e4 NA G A 225 NA FALSE NA NA 11
10 SRR25848… CP00… 6.40e4 NA G A 225 NA FALSE NA NA 18
# ℹ 330 more rows
# ℹ 17 more variables: VDB <dbl>, RPB <dbl>, MQB <dbl>, BQB <dbl>, MQSB <dbl>,
# SGB <dbl>, MQ0F <dbl>, ICB <lgl>, HOB <lgl>, AC <dbl>, AN <dbl>, DP4 <chr>,
# MQ <dbl>, Indiv <chr>, gt_PL <dbl>, gt_GT <dbl>, gt_GT_alleles <chr>R
# rows that have TRUE in the column INDEL
filter(variants, INDEL)
OUTPUT
# A tibble: 101 × 29
sample_id CHROM POS ID REF ALT QUAL FILTER INDEL IDV IMF DP
<chr> <chr> <dbl> <lgl> <chr> <chr> <dbl> <lgl> <lgl> <dbl> <dbl> <dbl>
1 SRR25848… CP00… 4.33e5 NA CTTT… CTTT… 64 NA TRUE 12 1 12
2 SRR25848… CP00… 4.74e5 NA CCGC CCGC… 228 NA TRUE 9 0.9 10
3 SRR25848… CP00… 2.10e6 NA ACAG… ACAG… 56 NA TRUE 2 0.667 3
4 SRR25848… CP00… 2.33e6 NA AT ATT 167 NA TRUE 7 1 7
5 SRR25848… CP00… 3.90e6 NA A AC 43.4 NA TRUE 2 1 2
6 SRR25848… CP00… 4.43e6 NA TGG T 228 NA TRUE 10 1 10
7 SRR25848… CP00… 1.48e5 NA AGGGG AGGG… 122 NA TRUE 8 1 8
8 SRR25848… CP00… 1.58e5 NA GTTT… GTTT… 19.5 NA TRUE 6 1 6
9 SRR25848… CP00… 1.73e5 NA CAA CA 180 NA TRUE 11 1 11
10 SRR25848… CP00… 1.75e5 NA GAA GA 194 NA TRUE 10 1 10
# ℹ 91 more rows
# ℹ 17 more variables: VDB <dbl>, RPB <dbl>, MQB <dbl>, BQB <dbl>, MQSB <dbl>,
# SGB <dbl>, MQ0F <dbl>, ICB <lgl>, HOB <lgl>, AC <dbl>, AN <dbl>, DP4 <chr>,
# MQ <dbl>, Indiv <chr>, gt_PL <dbl>, gt_GT <dbl>, gt_GT_alleles <chr>R
# rows that don't have missing data in the IDV column
filter(variants, !is.na(IDV))
OUTPUT
# A tibble: 101 × 29
sample_id CHROM POS ID REF ALT QUAL FILTER INDEL IDV IMF DP
<chr> <chr> <dbl> <lgl> <chr> <chr> <dbl> <lgl> <lgl> <dbl> <dbl> <dbl>
1 SRR25848… CP00… 4.33e5 NA CTTT… CTTT… 64 NA TRUE 12 1 12
2 SRR25848… CP00… 4.74e5 NA CCGC CCGC… 228 NA TRUE 9 0.9 10
3 SRR25848… CP00… 2.10e6 NA ACAG… ACAG… 56 NA TRUE 2 0.667 3
4 SRR25848… CP00… 2.33e6 NA AT ATT 167 NA TRUE 7 1 7
5 SRR25848… CP00… 3.90e6 NA A AC 43.4 NA TRUE 2 1 2
6 SRR25848… CP00… 4.43e6 NA TGG T 228 NA TRUE 10 1 10
7 SRR25848… CP00… 1.48e5 NA AGGGG AGGG… 122 NA TRUE 8 1 8
8 SRR25848… CP00… 1.58e5 NA GTTT… GTTT… 19.5 NA TRUE 6 1 6
9 SRR25848… CP00… 1.73e5 NA CAA CA 180 NA TRUE 11 1 11
10 SRR25848… CP00… 1.75e5 NA GAA GA 194 NA TRUE 10 1 10
# ℹ 91 more rows
# ℹ 17 more variables: VDB <dbl>, RPB <dbl>, MQB <dbl>, BQB <dbl>, MQSB <dbl>,
# SGB <dbl>, MQ0F <dbl>, ICB <lgl>, HOB <lgl>, AC <dbl>, AN <dbl>, DP4 <chr>,
# MQ <dbl>, Indiv <chr>, gt_PL <dbl>, gt_GT <dbl>, gt_GT_alleles <chr>We have a column titled “QUAL”. This is a Phred-scaled confidence
score that a polymorphism exists at this position given the sequencing
data. Lower QUAL scores indicate low probability of a polymorphism
existing at that site. filter() can be useful for selecting
mutations that have a QUAL score above a certain threshold:
R
# rows with QUAL values greater than or equal to 100
filter(variants, QUAL >= 100)
OUTPUT
# A tibble: 666 × 29
sample_id CHROM POS ID REF ALT QUAL FILTER INDEL IDV IMF DP
<chr> <chr> <dbl> <lgl> <chr> <chr> <dbl> <lgl> <lgl> <dbl> <dbl> <dbl>
1 SRR25848… CP00… 2.82e5 NA G T 217 NA FALSE NA NA 10
2 SRR25848… CP00… 4.74e5 NA CCGC CCGC… 228 NA TRUE 9 0.9 10
3 SRR25848… CP00… 6.49e5 NA C T 210 NA FALSE NA NA 10
4 SRR25848… CP00… 1.33e6 NA C A 178 NA FALSE NA NA 8
5 SRR25848… CP00… 1.73e6 NA G A 225 NA FALSE NA NA 11
6 SRR25848… CP00… 2.33e6 NA AT ATT 167 NA TRUE 7 1 7
7 SRR25848… CP00… 2.41e6 NA A C 104 NA FALSE NA NA 9
8 SRR25848… CP00… 2.45e6 NA A C 225 NA FALSE NA NA 20
9 SRR25848… CP00… 2.67e6 NA A T 225 NA FALSE NA NA 19
10 SRR25848… CP00… 3.00e6 NA G A 225 NA FALSE NA NA 15
# ℹ 656 more rows
# ℹ 17 more variables: VDB <dbl>, RPB <dbl>, MQB <dbl>, BQB <dbl>, MQSB <dbl>,
# SGB <dbl>, MQ0F <dbl>, ICB <lgl>, HOB <lgl>, AC <dbl>, AN <dbl>, DP4 <chr>,
# MQ <dbl>, Indiv <chr>, gt_PL <dbl>, gt_GT <dbl>, gt_GT_alleles <chr>filter() allows you to combine multiple conditions. You
can separate them using a , as arguments to the function,
they will be combined using the & (AND) logical
operator. If you need to use the | (OR) logical operator,
you can specify it explicitly:
R
# this is equivalent to:
# filter(variants, sample_id == "SRR2584863" & QUAL >= 100)
filter(variants, sample_id == "SRR2584863", QUAL >= 100)
OUTPUT
# A tibble: 19 × 29
sample_id CHROM POS ID REF ALT QUAL FILTER INDEL IDV IMF DP
<chr> <chr> <dbl> <lgl> <chr> <chr> <dbl> <lgl> <lgl> <dbl> <dbl> <dbl>
1 SRR25848… CP00… 2.82e5 NA G T 217 NA FALSE NA NA 10
2 SRR25848… CP00… 4.74e5 NA CCGC CCGC… 228 NA TRUE 9 0.9 10
3 SRR25848… CP00… 6.49e5 NA C T 210 NA FALSE NA NA 10
4 SRR25848… CP00… 1.33e6 NA C A 178 NA FALSE NA NA 8
5 SRR25848… CP00… 1.73e6 NA G A 225 NA FALSE NA NA 11
6 SRR25848… CP00… 2.33e6 NA AT ATT 167 NA TRUE 7 1 7
7 SRR25848… CP00… 2.41e6 NA A C 104 NA FALSE NA NA 9
8 SRR25848… CP00… 2.45e6 NA A C 225 NA FALSE NA NA 20
9 SRR25848… CP00… 2.67e6 NA A T 225 NA FALSE NA NA 19
10 SRR25848… CP00… 3.00e6 NA G A 225 NA FALSE NA NA 15
11 SRR25848… CP00… 3.34e6 NA A C 211 NA FALSE NA NA 10
12 SRR25848… CP00… 3.40e6 NA C A 225 NA FALSE NA NA 14
13 SRR25848… CP00… 3.48e6 NA A G 200 NA FALSE NA NA 9
14 SRR25848… CP00… 3.49e6 NA A C 225 NA FALSE NA NA 13
15 SRR25848… CP00… 3.91e6 NA G T 225 NA FALSE NA NA 10
16 SRR25848… CP00… 4.10e6 NA A G 225 NA FALSE NA NA 16
17 SRR25848… CP00… 4.20e6 NA A C 225 NA FALSE NA NA 11
18 SRR25848… CP00… 4.43e6 NA TGG T 228 NA TRUE 10 1 10
19 SRR25848… CP00… 4.62e6 NA A C 185 NA FALSE NA NA 9
# ℹ 17 more variables: VDB <dbl>, RPB <dbl>, MQB <dbl>, BQB <dbl>, MQSB <dbl>,
# SGB <dbl>, MQ0F <dbl>, ICB <lgl>, HOB <lgl>, AC <dbl>, AN <dbl>, DP4 <chr>,
# MQ <dbl>, Indiv <chr>, gt_PL <dbl>, gt_GT <dbl>, gt_GT_alleles <chr>R
# using `|` logical operator
filter(variants, sample_id == "SRR2584863", (MQ >= 50 | QUAL >= 100))
OUTPUT
# A tibble: 23 × 29
sample_id CHROM POS ID REF ALT QUAL FILTER INDEL IDV IMF
<chr> <chr> <dbl> <lgl> <chr> <chr> <dbl> <lgl> <lgl> <dbl> <dbl>
1 SRR2584863 CP000819… 9.97e3 NA T G 91 NA FALSE NA NA
2 SRR2584863 CP000819… 2.82e5 NA G T 217 NA FALSE NA NA
3 SRR2584863 CP000819… 4.33e5 NA CTTT… CTTT… 64 NA TRUE 12 1
4 SRR2584863 CP000819… 4.74e5 NA CCGC CCGC… 228 NA TRUE 9 0.9
5 SRR2584863 CP000819… 6.49e5 NA C T 210 NA FALSE NA NA
6 SRR2584863 CP000819… 1.33e6 NA C A 178 NA FALSE NA NA
7 SRR2584863 CP000819… 1.73e6 NA G A 225 NA FALSE NA NA
8 SRR2584863 CP000819… 2.10e6 NA ACAG… ACAG… 56 NA TRUE 2 0.667
9 SRR2584863 CP000819… 2.33e6 NA AT ATT 167 NA TRUE 7 1
10 SRR2584863 CP000819… 2.41e6 NA A C 104 NA FALSE NA NA
# ℹ 13 more rows
# ℹ 18 more variables: DP <dbl>, VDB <dbl>, RPB <dbl>, MQB <dbl>, BQB <dbl>,
# MQSB <dbl>, SGB <dbl>, MQ0F <dbl>, ICB <lgl>, HOB <lgl>, AC <dbl>,
# AN <dbl>, DP4 <chr>, MQ <dbl>, Indiv <chr>, gt_PL <dbl>, gt_GT <dbl>,
# gt_GT_alleles <chr>R
filter(variants, POS >= 1e6 & POS <= 2e6, QUAL > 200, !INDEL)
OUTPUT
# A tibble: 77 × 29
sample_id CHROM POS ID REF ALT QUAL FILTER INDEL IDV IMF DP
<chr> <chr> <dbl> <lgl> <chr> <chr> <dbl> <lgl> <lgl> <dbl> <dbl> <dbl>
1 SRR25848… CP00… 1.73e6 NA G A 225 NA FALSE NA NA 11
2 SRR25848… CP00… 1.00e6 NA A G 225 NA FALSE NA NA 15
3 SRR25848… CP00… 1.02e6 NA A G 225 NA FALSE NA NA 12
4 SRR25848… CP00… 1.06e6 NA C T 225 NA FALSE NA NA 17
5 SRR25848… CP00… 1.06e6 NA A G 206 NA FALSE NA NA 9
6 SRR25848… CP00… 1.07e6 NA G T 225 NA FALSE NA NA 11
7 SRR25848… CP00… 1.07e6 NA T C 225 NA FALSE NA NA 12
8 SRR25848… CP00… 1.10e6 NA C T 225 NA FALSE NA NA 15
9 SRR25848… CP00… 1.11e6 NA C T 212 NA FALSE NA NA 9
10 SRR25848… CP00… 1.11e6 NA A G 225 NA FALSE NA NA 14
# ℹ 67 more rows
# ℹ 17 more variables: VDB <dbl>, RPB <dbl>, MQB <dbl>, BQB <dbl>, MQSB <dbl>,
# SGB <dbl>, MQ0F <dbl>, ICB <lgl>, HOB <lgl>, AC <dbl>, AN <dbl>, DP4 <chr>,
# MQ <dbl>, Indiv <chr>, gt_PL <dbl>, gt_GT <dbl>, gt_GT_alleles <chr>Pipes
But what if you wanted to select and filter? We can do this with
pipes. Pipes, are a fairly recent addition to R. Pipes let you take the
output of one function and send it directly to the next, which is useful
when you need to many things to the same data set. It was possible to do
this before pipes were added to R, but it was much messier and more
difficult. Pipes in R look like %>% and are made
available via the magrittr package, which is installed as
part of dplyr. If you use RStudio, you can type the pipe
with Ctrl + Shift + M if you’re using a
PC, or Cmd + Shift + M if you’re using
a Mac.
R
variants %>%
filter(sample_id == "SRR2584863") %>%
select(REF, ALT, DP)
OUTPUT
# A tibble: 25 × 3
REF ALT DP
<chr> <chr> <dbl>
1 T G 4
2 G T 6
3 G T 10
4 CTTTTTTT CTTTTTTTT 12
5 CCGC CCGCGC 10
6 C T 10
7 C A 8
8 G A 11
9 ACAGCCAGCCAGCCAGCCAGCCAGCCAGCCAG ACAGCCAGCCAGCCAGCCAGCCAGCCAGCCAGCCAGC… 3
10 AT ATT 7
# ℹ 15 more rowsIn the above code, we use the pipe to send the variants
data set first through filter(), to keep rows where
sample_id matches a particular sample, and then through
select() to keep only the REF,
ALT, and DP columns. Since %>%
takes the object on its left and passes it as the first argument to the
function on its right, we don’t need to explicitly include the data
frame as an argument to the filter() and
select() functions any more.
Some may find it helpful to read the pipe like the word “then”. For
instance, in the above example, we took the data frame
variants, then we filtered for rows
where sample_id was SRR2584863, then we
selected the REF, ALT, and
DP columns, then we showed only the first six
rows. The dplyr functions by themselves
are somewhat simple, but by combining them into linear workflows with
the pipe, we can accomplish more complex manipulations of data
frames.
If we want to create a new object with this smaller version of the data we can do so by assigning it a new name:
R
SRR2584863_variants <- variants %>%
filter(sample_id == "SRR2584863") %>%
select(REF, ALT, DP)
This new object includes all of the data from this sample. Let’s look at just the first six rows to confirm it’s what we want:
R
SRR2584863_variants
OUTPUT
# A tibble: 25 × 3
REF ALT DP
<chr> <chr> <dbl>
1 T G 4
2 G T 6
3 G T 10
4 CTTTTTTT CTTTTTTTT 12
5 CCGC CCGCGC 10
6 C T 10
7 C A 8
8 G A 11
9 ACAGCCAGCCAGCCAGCCAGCCAGCCAGCCAG ACAGCCAGCCAGCCAGCCAGCCAGCCAGCCAGCCAGC… 3
10 AT ATT 7
# ℹ 15 more rowsSimilar to head() and tail() functions, we
can also look at the first or last six rows using tidyverse function
slice(). Slice is a more versatile function that allows
users to specify a range to view:
R
SRR2584863_variants %>% slice(1:6)
OUTPUT
# A tibble: 6 × 3
REF ALT DP
<chr> <chr> <dbl>
1 T G 4
2 G T 6
3 G T 10
4 CTTTTTTT CTTTTTTTT 12
5 CCGC CCGCGC 10
6 C T 10R
SRR2584863_variants %>% slice(10:25)
OUTPUT
# A tibble: 16 × 3
REF ALT DP
<chr> <chr> <dbl>
1 AT ATT 7
2 A C 9
3 A C 20
4 G T 12
5 A T 19
6 G A 15
7 A C 10
8 C A 14
9 A G 9
10 A C 13
11 A AC 2
12 G T 10
13 A G 16
14 A C 11
15 TGG T 10
16 A C 9R
variants %>%
filter(sample_id == "SRR2584863" & DP >= 10) %>%
slice(5:11) %>%
select(sample_id, DP, REF, ALT, POS)
OUTPUT
# A tibble: 7 × 5
sample_id DP REF ALT POS
<chr> <dbl> <chr> <chr> <dbl>
1 SRR2584863 11 G A 1733343
2 SRR2584863 20 A C 2446984
3 SRR2584863 12 G T 2618472
4 SRR2584863 19 A T 2665639
5 SRR2584863 15 G A 2999330
6 SRR2584863 10 A C 3339313
7 SRR2584863 14 C A 3401754Mutate
Frequently you’ll want to create new columns based on the values in
existing columns, for example to do unit conversions or find the ratio
of values in two columns. For this we’ll use the dplyr
function mutate().
For example, we can convert the polymorphism confidence value QUAL to a probability value according to the formula:
Probability = 1- 10 ^ -(QUAL/10)
We can use mutate to add a column (POLPROB)
to our variants data frame that shows the probability of a
polymorphism at that site given the data.
R
variants %>%
mutate(POLPROB = 1 - (10 ^ -(QUAL/10)))
OUTPUT
# A tibble: 801 × 30
sample_id CHROM POS ID REF ALT QUAL FILTER INDEL IDV IMF
<chr> <chr> <dbl> <lgl> <chr> <chr> <dbl> <lgl> <lgl> <dbl> <dbl>
1 SRR2584863 CP000819… 9.97e3 NA T G 91 NA FALSE NA NA
2 SRR2584863 CP000819… 2.63e5 NA G T 85 NA FALSE NA NA
3 SRR2584863 CP000819… 2.82e5 NA G T 217 NA FALSE NA NA
4 SRR2584863 CP000819… 4.33e5 NA CTTT… CTTT… 64 NA TRUE 12 1
5 SRR2584863 CP000819… 4.74e5 NA CCGC CCGC… 228 NA TRUE 9 0.9
6 SRR2584863 CP000819… 6.49e5 NA C T 210 NA FALSE NA NA
7 SRR2584863 CP000819… 1.33e6 NA C A 178 NA FALSE NA NA
8 SRR2584863 CP000819… 1.73e6 NA G A 225 NA FALSE NA NA
9 SRR2584863 CP000819… 2.10e6 NA ACAG… ACAG… 56 NA TRUE 2 0.667
10 SRR2584863 CP000819… 2.33e6 NA AT ATT 167 NA TRUE 7 1
# ℹ 791 more rows
# ℹ 19 more variables: DP <dbl>, VDB <dbl>, RPB <dbl>, MQB <dbl>, BQB <dbl>,
# MQSB <dbl>, SGB <dbl>, MQ0F <dbl>, ICB <lgl>, HOB <lgl>, AC <dbl>,
# AN <dbl>, DP4 <chr>, MQ <dbl>, Indiv <chr>, gt_PL <dbl>, gt_GT <dbl>,
# gt_GT_alleles <chr>, POLPROB <dbl>R
variants %>%
mutate(POLPROB = 1 - 10 ^ -(QUAL/10)) %>%
select(sample_id, POS, QUAL, POLPROB)
OUTPUT
# A tibble: 801 × 4
sample_id POS QUAL POLPROB
<chr> <dbl> <dbl> <dbl>
1 SRR2584863 9972 91 1.00
2 SRR2584863 263235 85 1.00
3 SRR2584863 281923 217 1
4 SRR2584863 433359 64 1.00
5 SRR2584863 473901 228 1
6 SRR2584863 648692 210 1
7 SRR2584863 1331794 178 1
8 SRR2584863 1733343 225 1
9 SRR2584863 2103887 56 1.00
10 SRR2584863 2333538 167 1
# ℹ 791 more rowsgroup_by() and summarize() functions
Many data analysis tasks can be approached using the
“split-apply-combine” paradigm: split the data into groups, apply some
analysis to each group, and then combine the results. dplyr
makes this very easy through the use of the group_by()
function, which splits the data into groups.
We can use group_by() to tally the number of mutations
detected in each sample using the function tally():
R
variants %>%
group_by(sample_id) %>%
tally()
OUTPUT
# A tibble: 3 × 2
sample_id n
<chr> <int>
1 SRR2584863 25
2 SRR2584866 766
3 SRR2589044 10Since counting or tallying values is a common use case for
group_by(), an alternative function was created to bypasses
group_by() using the function count():
R
variants %>%
count(sample_id)
OUTPUT
# A tibble: 3 × 2
sample_id n
<chr> <int>
1 SRR2584863 25
2 SRR2584866 766
3 SRR2589044 10R
variants %>%
count(INDEL)
OUTPUT
# A tibble: 2 × 2
INDEL n
<lgl> <int>
1 FALSE 700
2 TRUE 101When the data is grouped, summarize() can be used to
collapse each group into a single-row summary. summarize()
does this by applying an aggregating or summary function to each
group.
It can be a bit tricky at first, but we can imagine physically splitting the data frame by groups and applying a certain function to summarize the data.
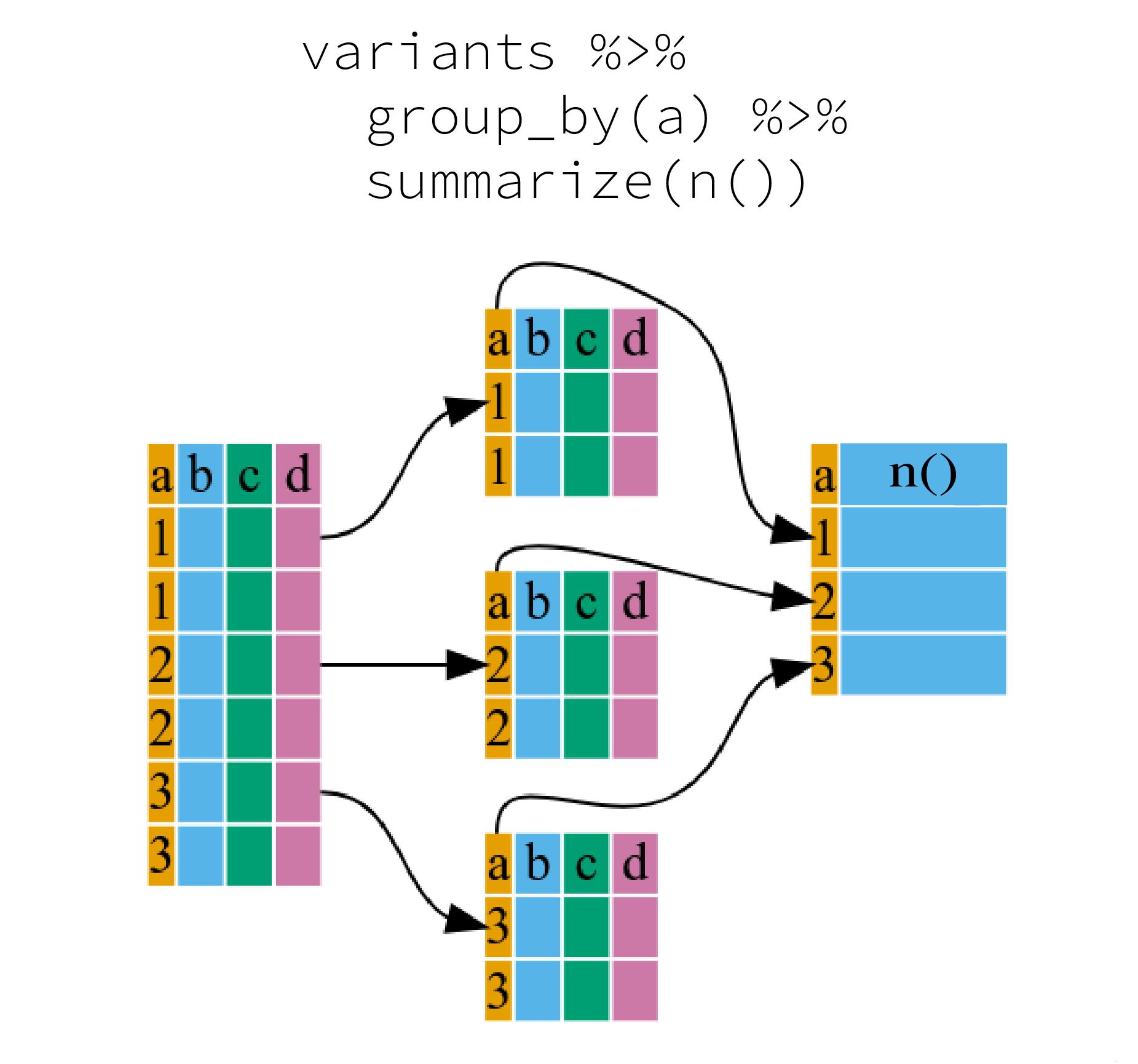
We can also apply many other functions to individual columns to get
other summary statistics. For example,we can use built-in functions like
mean(), median(), min(), and
max(). These are called “built-in functions” because they
come with R and don’t require that you install any additional packages.
By default, all R functions operating on vectors that contains
missing data will return NA. It’s a way to make sure that users
know they have missing data, and make a conscious decision on how to
deal with it. When dealing with simple statistics like the mean, the
easiest way to ignore NA (the missing data) is to use
na.rm = TRUE (rm stands for remove).
So to view the mean, median, maximum, and minimum filtered depth
(DP) for each sample:
R
variants %>%
group_by(sample_id) %>%
summarize(
mean_DP = mean(DP),
median_DP = median(DP),
min_DP = min(DP),
max_DP = max(DP))
OUTPUT
# A tibble: 3 × 5
sample_id mean_DP median_DP min_DP max_DP
<chr> <dbl> <dbl> <dbl> <dbl>
1 SRR2584863 10.4 10 2 20
2 SRR2584866 10.6 10 2 79
3 SRR2589044 9.3 9.5 3 16Reshaping data frames
It can sometimes be useful to transform the “long” tidy format, into
the wide format. This transformation can be done with the
pivot_wider() function provided by the tidyr
package (also part of the tidyverse).
pivot_wider() takes a data frame as the first argument,
and two arguments: the column name that will become the columns and the
column name that will become the cells in the wide data.
R
variants_wide <- variants %>%
group_by(sample_id, CHROM) %>%
summarize(mean_DP = mean(DP)) %>%
pivot_wider(names_from = sample_id, values_from = mean_DP)
OUTPUT
`summarise()` has grouped output by 'sample_id'. You can override using the
`.groups` argument.R
variants_wide
OUTPUT
# A tibble: 1 × 4
CHROM SRR2584863 SRR2584866 SRR2589044
<chr> <dbl> <dbl> <dbl>
1 CP000819.1 10.4 10.6 9.3The opposite operation of pivot_wider() is taken care by
pivot_longer(). We specify the names of the new columns,
and here add -CHROM as this column shouldn’t be affected by
the reshaping:
R
variants_wide %>%
pivot_longer(-CHROM, names_to = "sample_id", values_to = "mean_DP")
OUTPUT
# A tibble: 3 × 3
CHROM sample_id mean_DP
<chr> <chr> <dbl>
1 CP000819.1 SRR2584863 10.4
2 CP000819.1 SRR2584866 10.6
3 CP000819.1 SRR2589044 9.3Resources
Keypoints
- Use the
dplyrpackage to manipulate data frames. - Use
glimpse()to quickly look at your data frame. - Use
select()to choose variables from a data frame. - Use
filter()to choose data based on values. - Use
mutate()to create new variables. - Use
group_by()andsummarize()to work with subsets of data.
The figure was adapted from the Software Carpentry lesson, R for Reproducible Scientific Analysis↩︎
Content from Data Visualization with ggplot2
Last updated on 2023-08-15 | Edit this page
Estimated time 90 minutes
Overview
Questions
- What is ggplot2?
- What is mapping, and what is aesthetics?
- What is the process of creating a publication-quality plots with ggplot in R?
Objectives
- Describe the role of data, aesthetics, and geoms in ggplot functions.
- Choose the correct aesthetics and alter the geom parameters for a scatter plot, histogram, or box plot.
- Layer multiple geometries in a single plot.
- Customize plot scales, titles, themes, and fonts.
- Apply a facet to a plot.
- Apply additional ggplot2-compatible plotting libraries.
- Save a ggplot to a file.
- List several resources for getting help with ggplot.
- List several resources for creating informative scientific plots.
Introduction to ggplot2

ggplot2 is a plotting package that
makes it simple to create complex plots from data in a data frame. It
provides a more programmatic interface for specifying what variables to
plot, how they are displayed, and general visual properties. Therefore,
we only need minimal changes if the underlying data change or if we
decide to change from a bar plot to a scatter plot. This helps in
creating publication-quality plots with minimal amounts of adjustments
and tweaking.
The gg in “ggplot” stands for “Grammar of Graphics,” which is an elegant yet powerful way to describe the making of scientific plots. In short, the grammar of graphics breaks down every plot into a few components, namely, a dataset, a set of geoms (visual marks that represent the data points), and a coordinate system. You can imagine this is a grammar that gives unique names to each component appearing in a plot and conveys specific information about data. With ggplot, graphics are built step by step by adding new elements.
The idea of mapping is crucial in
ggplot. One familiar example is to map the
value of one variable in a dataset to \(x\) and the other to \(y\). However, we often encounter datasets
that include multiple (more than two) variables. In this case,
ggplot allows you to map those other variables
to visual marks such as color and
shape (aesthetics or
aes). One thing you may want to remember is the difference
between discrete and continuous
variables. Some aesthetics, such as the shape of dots, do not accept
continuous variables. If forced to do so, R will give an error. This is
easy to understand; we cannot create a continuum of shapes for a
variable, unlike, say, color.
Tip: when having doubts about whether a variable is
continuous
or discrete, a quick way to check is to use the summary()
function. Continuous variables have descriptive statistics but not the
discrete variables.
Installing tidyverse
ggplot2 belongs to the tidyverse
framework. Therefore, we will start with loading the package
tidyverse. If
tidyverse is not already installed, then
we need to install first. If it is already installed, then we can skip
the following step:
R
install.packages("tidyverse") # Installing tidyverse package, includes ggplot2 and other packages such as dplyr, readr, tidyr
Now, let’s load the tidyverse package:
R
library(tidyverse)
OUTPUT
── Attaching core tidyverse packages ──────────────────────── tidyverse 2.0.0 ──
✔ dplyr 1.1.2 ✔ readr 2.1.4
✔ forcats 1.0.0 ✔ stringr 1.5.0
✔ ggplot2 3.4.2 ✔ tibble 3.2.1
✔ lubridate 1.9.2 ✔ tidyr 1.3.0
✔ purrr 1.0.1
── Conflicts ────────────────────────────────────────── tidyverse_conflicts() ──
✖ dplyr::filter() masks stats::filter()
✖ dplyr::lag() masks stats::lag()
ℹ Use the conflicted package (<http://conflicted.r-lib.org/>) to force all conflicts to become errorsAs we can see from above output ggplot2
has been already loaded along with other packages as part of the
tidyverse framework.
Loading the dataset
R
variants = read_csv("https://raw.githubusercontent.com/naupaka/vcfr-for-data-carpentry-draft/main/output/combined_tidy_vcf.csv")
OUTPUT
Rows: 801 Columns: 29
── Column specification ────────────────────────────────────────────────────────
Delimiter: ","
chr (7): sample_id, CHROM, REF, ALT, DP4, Indiv, gt_GT_alleles
dbl (16): POS, QUAL, IDV, IMF, DP, VDB, RPB, MQB, BQB, MQSB, SGB, MQ0F, AC, ...
num (1): gt_PL
lgl (5): ID, FILTER, INDEL, ICB, HOB
ℹ Use `spec()` to retrieve the full column specification for this data.
ℹ Specify the column types or set `show_col_types = FALSE` to quiet this message.Explore the structure (types of columns and number of rows)
of the dataset using dplyr’s glimpse()
(for more info, see the Data
Wrangling and Analyses with Tidyverse episode)
R
glimpse(variants) # Show a snapshot of the rows and columns
OUTPUT
Rows: 801
Columns: 29
$ sample_id <chr> "SRR2584863", "SRR2584863", "SRR2584863", "SRR2584863", …
$ CHROM <chr> "CP000819.1", "CP000819.1", "CP000819.1", "CP000819.1", …
$ POS <dbl> 9972, 263235, 281923, 433359, 473901, 648692, 1331794, 1…
$ ID <lgl> NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, …
$ REF <chr> "T", "G", "G", "CTTTTTTT", "CCGC", "C", "C", "G", "ACAGC…
$ ALT <chr> "G", "T", "T", "CTTTTTTTT", "CCGCGC", "T", "A", "A", "AC…
$ QUAL <dbl> 91.0000, 85.0000, 217.0000, 64.0000, 228.0000, 210.0000,…
$ FILTER <lgl> NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, …
$ INDEL <lgl> FALSE, FALSE, FALSE, TRUE, TRUE, FALSE, FALSE, FALSE, TR…
$ IDV <dbl> NA, NA, NA, 12, 9, NA, NA, NA, 2, 7, NA, NA, NA, NA, NA,…
$ IMF <dbl> NA, NA, NA, 1.000000, 0.900000, NA, NA, NA, 0.666667, 1.…
$ DP <dbl> 4, 6, 10, 12, 10, 10, 8, 11, 3, 7, 9, 20, 12, 19, 15, 10…
$ VDB <dbl> 0.0257451, 0.0961330, 0.7740830, 0.4777040, 0.6595050, 0…
$ RPB <dbl> NA, 1.000000, NA, NA, NA, NA, NA, NA, NA, NA, 0.900802, …
$ MQB <dbl> NA, 1.0000000, NA, NA, NA, NA, NA, NA, NA, NA, 0.1501340…
$ BQB <dbl> NA, 1.000000, NA, NA, NA, NA, NA, NA, NA, NA, 0.750668, …
$ MQSB <dbl> NA, NA, 0.974597, 1.000000, 0.916482, 0.916482, 0.900802…
$ SGB <dbl> -0.556411, -0.590765, -0.662043, -0.676189, -0.662043, -…
$ MQ0F <dbl> 0.000000, 0.166667, 0.000000, 0.000000, 0.000000, 0.0000…
$ ICB <lgl> NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, …
$ HOB <lgl> NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, NA, …
$ AC <dbl> 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1,…
$ AN <dbl> 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1,…
$ DP4 <chr> "0,0,0,4", "0,1,0,5", "0,0,4,5", "0,1,3,8", "1,0,2,7", "…
$ MQ <dbl> 60, 33, 60, 60, 60, 60, 60, 60, 60, 60, 25, 60, 10, 60, …
$ Indiv <chr> "/home/dcuser/dc_workshop/results/bam/SRR2584863.aligned…
$ gt_PL <dbl> 1210, 1120, 2470, 910, 2550, 2400, 2080, 2550, 11128, 19…
$ gt_GT <dbl> 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1, 1,…
$ gt_GT_alleles <chr> "G", "T", "T", "CTTTTTTTT", "CCGCGC", "T", "A", "A", "AC…Alternatively, we can display the first a few rows (vertically) of
the table using head():
R
head(variants)
| sample_id | CHROM | POS | ID | REF | ALT | QUAL | FILTER | INDEL | IDV | IMF | DP | VDB | RPB | MQB | BQB | MQSB | SGB | MQ0F | ICB | HOB | AC | AN | DP4 | MQ | Indiv | gt_PL | gt_GT | gt_GT_alleles |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SRR2584863 | CP000819.1 | 9972 | NA | T | G | 91 | NA | FALSE | NA | NA | 4 | 0.0257451 | NA | NA | NA | NA | -0.556411 | 0.000000 | NA | NA | 1 | 1 | 0,0,0,4 | 60 | /home/dcuser/dc_workshop/results/bam/SRR2584863.aligned.sorted.bam | 1210 | 1 | G |
| SRR2584863 | CP000819.1 | 263235 | NA | G | T | 85 | NA | FALSE | NA | NA | 6 | 0.0961330 | 1 | 1 | 1 | NA | -0.590765 | 0.166667 | NA | NA | 1 | 1 | 0,1,0,5 | 33 | /home/dcuser/dc_workshop/results/bam/SRR2584863.aligned.sorted.bam | 1120 | 1 | T |
| SRR2584863 | CP000819.1 | 281923 | NA | G | T | 217 | NA | FALSE | NA | NA | 10 | 0.7740830 | NA | NA | NA | 0.974597 | -0.662043 | 0.000000 | NA | NA | 1 | 1 | 0,0,4,5 | 60 | /home/dcuser/dc_workshop/results/bam/SRR2584863.aligned.sorted.bam | 2470 | 1 | T |
| SRR2584863 | CP000819.1 | 433359 | NA | CTTTTTTT | CTTTTTTTT | 64 | NA | TRUE | 12 | 1.0 | 12 | 0.4777040 | NA | NA | NA | 1.000000 | -0.676189 | 0.000000 | NA | NA | 1 | 1 | 0,1,3,8 | 60 | /home/dcuser/dc_workshop/results/bam/SRR2584863.aligned.sorted.bam | 910 | 1 | CTTTTTTTT |
| SRR2584863 | CP000819.1 | 473901 | NA | CCGC | CCGCGC | 228 | NA | TRUE | 9 | 0.9 | 10 | 0.6595050 | NA | NA | NA | 0.916482 | -0.662043 | 0.000000 | NA | NA | 1 | 1 | 1,0,2,7 | 60 | /home/dcuser/dc_workshop/results/bam/SRR2584863.aligned.sorted.bam | 2550 | 1 | CCGCGC |
| SRR2584863 | CP000819.1 | 648692 | NA | C | T | 210 | NA | FALSE | NA | NA | 10 | 0.2680140 | NA | NA | NA | 0.916482 | -0.670168 | 0.000000 | NA | NA | 1 | 1 | 0,0,7,3 | 60 | /home/dcuser/dc_workshop/results/bam/SRR2584863.aligned.sorted.bam | 2400 | 1 | T |
ggplot2 functions like data in the
long format, i.e., a column for every dimension
(variable), and a row for every observation. Well-structured data will
save you time when making figures with
ggplot2
ggplot2 graphics are built step-by-step
by adding new elements. Adding layers in this fashion allows for
extensive flexibility and customization of plots, and more equally
important the readability of the code.
To build a ggplot, we will use the following basic template that can be used for different types of plots:
R
ggplot(data = <DATA>, mapping = aes(<MAPPINGS>)) + <GEOM_FUNCTION>()- use the
ggplot()function and bind the plot to a specific data frame using thedataargument
R
ggplot(data = variants)
- define a mapping (using the aesthetic (
aes) function), by selecting the variables to be plotted and specifying how to present them in the graph, e.g. as x and y positions or characteristics such as size, shape, color, etc.
R
ggplot(data = variants, aes(x = POS, y = DP))
- add ‘geoms’ – graphical representations of the data in the plot
(points, lines, bars).
ggplot2offers many different geoms; we will use some common ones today, including:-
geom_point()for scatter plots, dot plots, etc. -
geom_boxplot()for, well, boxplots! -
geom_line()for trend lines, time series, etc.
-
To add a geom to the plot use the + operator. Because we
have two continuous variables, let’s use geom_point()
(i.e., a scatter plot) first:
R
ggplot(data = variants, aes(x = POS, y = DP)) +
geom_point()
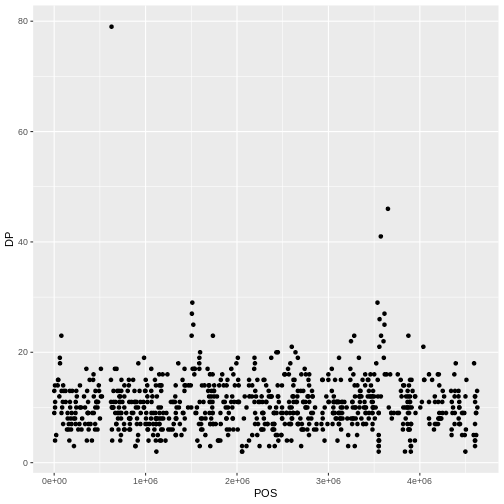
The + in the ggplot2
package is particularly useful because it allows you to modify existing
ggplot objects. This means you can easily set up plot
templates and conveniently explore different types of plots, so the
above plot can also be generated with code like this:
R
# Assign plot to a variable
coverage_plot <- ggplot(data = variants, aes(x = POS, y = DP))
# Draw the plot
coverage_plot +
geom_point()
Notes
- Anything you put in the
ggplot()function can be seen by any geom layers that you add (i.e., these are universal plot settings). This includes the x- and y-axis mapping you set up inaes(). - You can also specify mappings for a given geom independently of the
mappings defined globally in the
ggplot()function. - The
+sign used to add new layers must be placed at the end of the line containing the previous layer. If, instead, the+sign is added at the beginning of the line containing the new layer,ggplot2will not add the new layer and will return an error message.
R
# This is the correct syntax for adding layers
coverage_plot +
geom_point()
# This will not add the new layer and will return an error message
coverage_plot
+ geom_point()
Building your plots iteratively
Building plots with ggplot2 is
typically an iterative process. We start by defining the dataset we’ll
use, lay out the axes, and choose a geom:
R
ggplot(data = variants, aes(x = POS, y = DP)) +
geom_point()
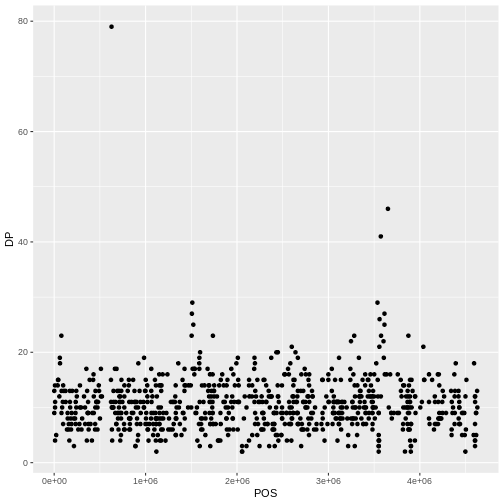
Then, we start modifying this plot to extract more information from
it. For instance, we can add transparency (alpha) to avoid
over-plotting:
R
ggplot(data = variants, aes(x = POS, y = DP)) +
geom_point(alpha = 0.5)
We can also add colors for all the points:
R
ggplot(data = variants, aes(x = POS, y = DP)) +
geom_point(alpha = 0.5, color = "blue")
Or to color each species in the plot differently, you could use a
vector as an input to the argument color.
ggplot2 will provide a different color
corresponding to different values in the vector. Here is an example
where we color with sample_id:
R
ggplot(data = variants, aes(x = POS, y = DP, color = sample_id)) +
geom_point(alpha = 0.5)
Notice that we can change the geom layer and colors will be still
determined by sample_id
R
ggplot(data = variants, aes(x = POS, y = DP, color = sample_id)) +
geom_point(alpha = 0.5)
To make our plot more readable, we can add axis labels:
R
ggplot(data = variants, aes(x = POS, y = DP, color = sample_id)) +
geom_point(alpha = 0.5) +
labs(x = "Base Pair Position",
y = "Read Depth (DP)")
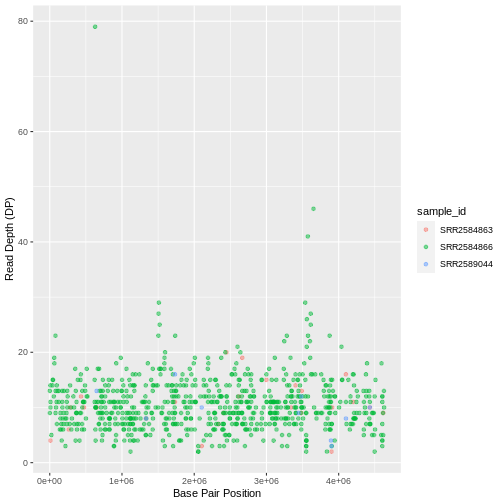
To add a main title to the plot, we use ggtitle():
R
ggplot(data = variants, aes(x = POS, y = DP, color = sample_id)) +
geom_point(alpha = 0.5) +
labs(x = "Base Pair Position",
y = "Read Depth (DP)") +
ggtitle("Read Depth vs. Position")
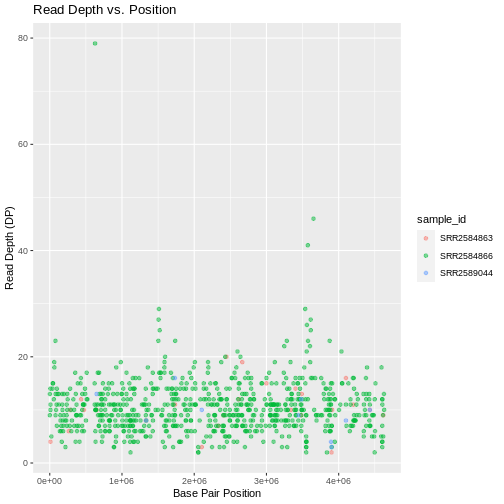
Now the figure is complete and ready to be exported and saved to a
file. This can be achieved easily using ggsave(),
which can write, by default, the most recent generated figure into
different formats (e.g., jpeg, png,
pdf) according to the file extension. So, for example, to
create a pdf version of the above figure with a dimension of \(6\times4\) inches:
R
ggsave ("depth.pdf", width = 6, height = 4)
If we check the current working directory, there should be a
newly created file called depth.pdf with the above
plot.
R
ggplot(data = variants, aes(x = POS, y = MQ, color = sample_id)) +
geom_point() +
labs(x = "Base Pair Position",
y = "Mapping Quality (MQ)")
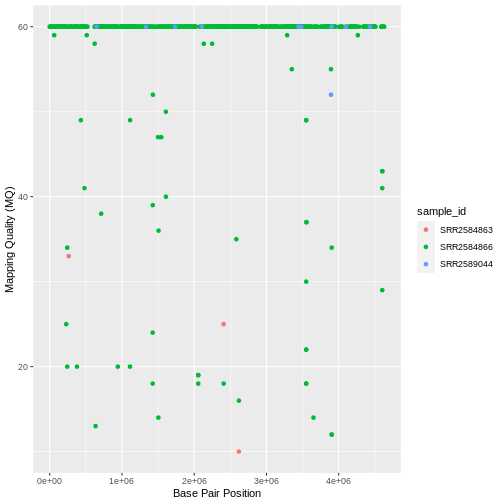
To further customize the plot, we can change the default font format:
R
ggplot(data = variants, aes(x = POS, y = DP, color = sample_id)) +
geom_point(alpha = 0.5) +
labs(x = "Base Pair Position",
y = "Read Depth (DP)") +
ggtitle("Read Depth vs. Position") +
theme(text = element_text(family = "Bookman"))
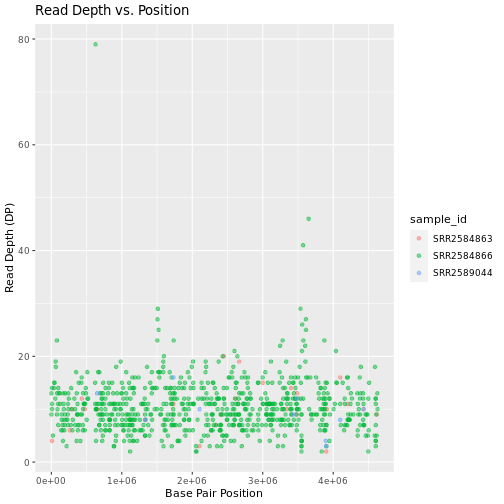
Faceting
ggplot2 has a special technique called
faceting that allows the user to split one plot into multiple
plots (panels) based on a factor (variable) included in the dataset. We
will use it to split our mapping quality plot into three panels, one for
each sample.
R
ggplot(data = variants, aes(x = POS, y = MQ, color = sample_id)) +
geom_point() +
labs(x = "Base Pair Position",
y = "Mapping Quality (MQ)") +
facet_grid(. ~ sample_id)
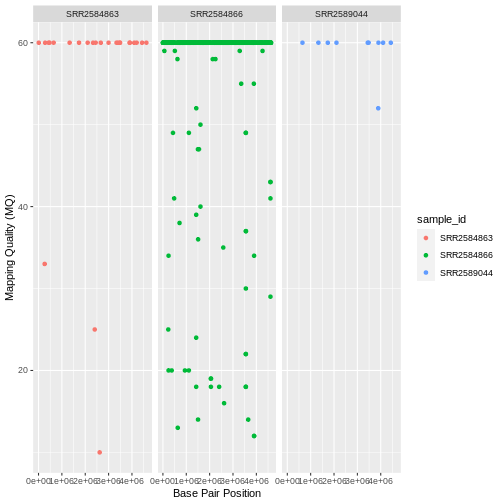
This looks okay, but it would be easier to read if the plot facets
were stacked vertically rather than horizontally. The
facet_grid geometry allows you to explicitly specify how
you want your plots to be arranged via formula notation
(rows ~ columns; the dot (.) indicates every
other variable in the data i.e., no faceting on that side of the
formula).
R
ggplot(data = variants, aes(x = POS, y = MQ, color = sample_id)) +
geom_point() +
labs(x = "Base Pair Position",
y = "Mapping Quality (MQ)") +
facet_grid(sample_id ~ .)
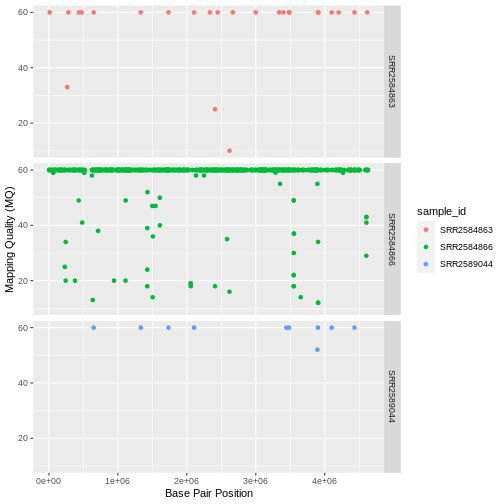
Usually plots with white background look more readable when printed.
We can set the background to white using the function theme_bw().
Additionally, you can remove the grid:
R
ggplot(data = variants, aes(x = POS, y = MQ, color = sample_id)) +
geom_point() +
labs(x = "Base Pair Position",
y = "Mapping Quality (MQ)") +
facet_grid(sample_id ~ .) +
theme_bw() +
theme(panel.grid = element_blank())
R
ggplot(data = variants, aes(x = POS, y = QUAL, color = sample_id)) +
geom_point() +
labs(x = "Base Pair Position",
y = "PHRED-sacled Quality (QUAL)") +
facet_grid(sample_id ~ .)
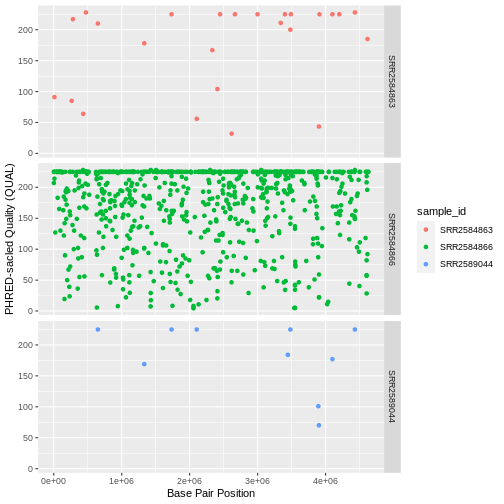
Barplots
We can create barplots using the geom_bar
geom. Let’s make a barplot showing the number of variants for each
sample that are indels.
R
ggplot(data = variants, aes(x = INDEL, fill = sample_id)) +
geom_bar() +
facet_grid(sample_id ~ .)
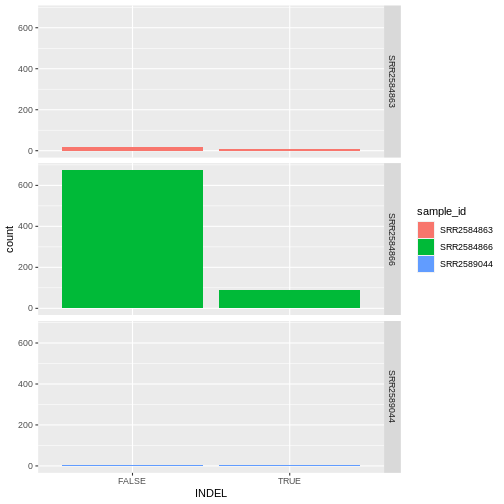
R
ggplot(data = variants, aes(x = INDEL, color = sample_id)) +
geom_bar(show.legend = F) +
facet_grid(sample_id ~ .)
Density
We can create density plots using the geom_density
geom that shows the distribution of of a variable in the dataset. Let’s
plot the distribution of DP
R
ggplot(data = variants, aes(x = DP)) +
geom_density()
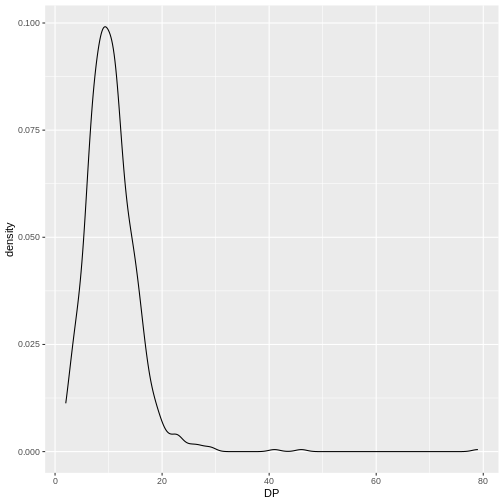
This plot tells us that the most of frequent DP (read
depth) for the variants is about 10 reads.
Challenge
Use geom_density
to plot the distribution of DP with a different fill for
each sample. Use a white background for the plot.
R
ggplot(data = variants, aes(x = DP, fill = sample_id)) +
geom_density(alpha = 0.5) +
theme_bw()
ggplot2 themes
In addition to theme_bw(),
which changes the plot background to white,
ggplot2 comes with several other themes
which can be useful to quickly change the look of your visualization.
The complete list of themes is available at https://ggplot2.tidyverse.org/reference/ggtheme.html.
theme_minimal() and theme_light() are popular,
and theme_void() can be useful as a starting point to
create a new hand-crafted theme.
The ggthemes
package provides a wide variety of options (including Microsoft Excel,
old
and new).
The ggplot2
extensions website provides a list of packages that extend the
capabilities of ggplot2, including
additional themes.
Challenge
With all of this information in hand, please take another five
minutes to either improve one of the plots generated in this exercise or
create a beautiful graph of your own. Use the RStudio ggplot2
cheat sheet for inspiration. Here are some ideas:
- See if you can change the size or shape of the plotting symbol.
- Can you find a way to change the name of the legend? What about its labels?
- Try using a different color palette (see the Cookbook for R.
More ggplot2 Plots
ggplot2 offers many more informative
and beautiful plots (geoms) of interest for biologists
(although not covered in this lesson) that are worth exploring, such
as
-
geom_tile(), for heatmaps, -
geom_jitter(), for strip charts, and -
geom_violin(), for violin plots
Resources
Content from Getting help with R
Last updated on 2023-08-15 | Edit this page
Estimated time 15 minutes
Overview
Questions
- How do I get help using R and RStudio?
Objectives
- Locate help for an R function using
?,??, andargs() - Check the version of R
- Be able to ask effective questions when searching for help on forums or using web searches
Getting help with R

No matter how much experience you have with R, you will find yourself needing help. There is no shame in researching how to do something in R, and most people will find themselves looking up how to do the same things that they “should know how to do” over and over again. Here are some tips to make this process as helpful and efficient as possible.
“Never memorize something that you can look up” -- A. Einstein
Finding help on Stackoverflow and Biostars
Two popular websites will be of great help with many R problems. For general R questions, Stack Overflow is probably the most popular online community for developers. If you start your question “How to do X in R” results from Stack Overflow are usually near the top of the list. For bioinformatics specific questions, Biostars is a popular online forum.
Tip: Asking for help using online forums:
- When searching for R help, look for answers with the r tag.
- Get an account; not required to view answers but to required to post
- Put in effort to check thoroughly before you post a question; folks get annoyed if you ask a very common question that has been answered multiple times
- Be careful. While forums are very helpful, you can’t know for sure if the advice you are getting is correct
- See the How to ask for R help blog post for more useful tips
Help people help you
Often, in order to duplicate the issue you are having, someone may need to see the data you are working with or verify the versions of R or R packages you are using. The following R functions will help with this:
You can check the version of R you are working with
using the sessionInfo() function. Actually, it is good to
save this information as part of your notes on any analysis you are
doing. When you run the same script that has worked fine a dozen times
before, looking back at these notes will remind you that you upgraded R
and forget to check your script.
sessionInfo()R version 3.2.3 (2015-12-10)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Ubuntu 14.04.3 LTS
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C LC_TIME=en_US.UTF-8
[4] LC_COLLATE=en_US.UTF-8 LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C LC_ADDRESS=C
[10] LC_TELEPHONE=C LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] stats graphics grDevices utils datasets methods base
loaded via a namespace (and not attached):
[1] tools_3.2.3 packrat_0.4.9-1Many times, there may be some issues with your data and the way it is
formatted. In that case, you may want to share that data with someone
else. However, you may not need to share the whole dataset; looking at a
subset of your 50,000 row, 10,000 column dataframe may be TMI (too much
information)! You can take an object you have in memory such as
dataframe (if you don’t know what this means yet, we will get to it!)
and save it to a file. In our example we will use the
dput() function on the iris dataframe which is
an example dataset that is installed in R:
dput(head(iris)) # iris is an example data.frame that comes with R
# the `head()` function just takes the first 6 lines of the iris datasetThis generates some output (below) which you will be better able to interpret after covering the other R lessons. This info would be helpful in understanding how the data is formatted and possibly revealing problematic issues.
structure(list(Sepal.Length = c(5.1, 4.9, 4.7, 4.6, 5, 5.4),
Sepal.Width = c(3.5, 3, 3.2, 3.1, 3.6, 3.9), Petal.Length = c(1.4,
1.4, 1.3, 1.5, 1.4, 1.7), Petal.Width = c(0.2, 0.2, 0.2,
0.2, 0.2, 0.4), Species = structure(c(1L, 1L, 1L, 1L, 1L,
1L), .Label = c("setosa", "versicolor", "virginica"), class = "factor")), .Names = c("Sepal.Length",
"Sepal.Width", "Petal.Length", "Petal.Width", "Species"), row.names = c(NA,
6L), class = "data.frame")Alternatively, you can also save objects in R memory to a file by
specifying the name of the object, in this case the iris
data frame, and passing a filename to the file=
argument.
R
saveRDS(iris, file="iris.rds") # By convention, we use the .rds file extension
Final FAQs on R
Finally, here are a few pieces of introductory R knowledge that are too good to pass up. While we won’t return to them in this course, we put them here because they come up commonly:
Do I need to click Run every time I want to run a script?
-
No. In fact, the most common shortcut key allows you to run a command (or any lines of the script that are highlighted):
- Windows execution shortcut: Ctrl+Enter
- Mac execution shortcut: Cmd(⌘)+Enter
To see a complete list of shortcuts, click on the Tools menu and select Keyboard Shortcuts Help
What’s with the brackets in R console output?
- R returns an index with your result. When your result contains multiple values, the number tells you what ordinal number begins the line, for example:
R
1:101 # generates the sequence of numbers from 1 to 101
OUTPUT
[1] 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18
[19] 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36
[37] 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54
[55] 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72
[73] 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90
[91] 91 92 93 94 95 96 97 98 99 100 101In the output above, [81] indicates that the first value
on that line is the 81st item in your result
Can I run my R script without RStudio?
- Yes, remember - RStudio is running R. You get to use lots of the enhancements RStudio provides, but R works independent of RStudio. See these tips for running your commands at the command line
Where else can I learn about RStudio?
- Check out the Help menu, especially “Cheatsheets” section
